In September 2001, the South Australian state-wide methicillin-resistant Staphylococcus aureus (MRSA) surveillance system was expanded to include three surveillance indicators namely: estimated MRSA burden, MRSA morbidity and estimated MRSA acquisition. The last two indicator rates have been stratified into intensive care unit (ICU) versus non-ICU. Between September 2001 and March 2002, state-wide MRSA burden rates (prevalence) ranged from 27.5 to 39.8 per 10,000 occupied bed days (OBDs). Acquisition rates ranged from 28.2 to 69.0 per 10,000 OBDs (ICU) and 6.3 to 10.1 per 10,000 OBDs (non-ICU). Morbidity rates ranged from 12.9 to 43.1 per 10,000 OBDs (ICU) and 3.0 to 5.0 per 10,000 OBDs (non-ICU). In association with the changes to surveillance indicators, a new monthly surveillance report was developed. Assuring confidentiality to individual contributing hospitals has been a major consideration in the development of the data collection system. Individual contributors have access only to their own indicator rates and pooled state-wide indicator rates. Contributing institutions are urged to use great caution if wishing to compare their own rates with state-wide rates. In particular, contributors are asked to take inter-institutional differences in MRSA burden and casemix complexity into account when making such comparisons. Commun Dis Intell 2003;27 Suppl:S92-S96.
Top of page
Introduction
Antibiotic-resistant organisms are now regarded as a significant and growing threat to public health worldwide.1 In October 2000, the Australian Commonwealth Government released its response to the report of the Joint Expert Technical Advisory Committee on Antibiotic Resistance (JETACAR) in which it supported surveillance of antibiotic-resistant organisms at the national level and at the level of individual states and territories.2
In response to the Commonwealth Government recommendations, the surveillance system for methicillin-resistant Staphylococcus aureus (MRSA) established in South Australia (December 2000), incorporating all the major public and private hospitals, was expanded in September 2001. The primary aim of this enhanced surveillance is to better understand the epidemiology of MRSA infection and colonisation in South Australia; this is seen as an important first step towards the development of effective local control programs.
Top of page
Methodology
Sixteen hospitals (eight public and eight private) currently contribute MRSA infection and colonisation data to the South Australian Department of Human Services (SADHS). The definitions and methodology used are based on those for multi-resistant organisms developed by the Australian Infection Control Association National Advisory Board, which are currently published in draft form.3 MRSA is defined as Staphylococcus aureus with acquired resistance to methicillin (usually reported as flucloxacillin- or oxacillin-resistant).
Each month the infection control practitioner responsible for MRSA surveillance at each contributing hospital supplies the following data about discharged patients:
Documented as infected or colonised
The number of patients with previously diagnosed MRSA. These patients may either have an infection (defined as an event associated with a sterile site isolate or an event associated with a non-sterile site clinical isolate where MRSA-specific antibiotic therapy was administered by a clinician) or colonisation (defined as a non-sterile site isolate and specific MRSA therapy has not been instituted).
The number of patients with newly-acquired, healthcare-related MRSA (either colonised or infected). Newly-acquired is defined as, not previously documented as infected or colonised, first detected more than 48 hours after admission or within 48 hours of discharge from the healthcare facility.
The number of newly-acquired healthcare-related MRSA infections (these may occur in patients previously known to be colonised) where the infection is first detected more than 48 hours after admission or within 48 hours of discharge from the healthcare facility.
For newly-acquired, healthcare-related MRSA cases (infected or colonised) the following patient data is also collected: medical record number; specialty unit (i.e., ICU or non-ICU); processing laboratory and laboratory identification number of isolate; infection status (i.e., infected or colonised); and infection site (i.e., sterile or non-sterile).
In order to calculate rates, the following hospital separations data are also captured each month (excluding day cases): the number of inpatient separations; the number of inpatient occupied bed days (OBDs); and the number of ICU OBDs.
Upon receipt of a hospital dataset at the SADHS, the data are manually entered into a Microsoft Excel 97 workbook. When all the MRSA and separations data for a month have been entered, the new data are then processed to calculate the following three rates, firstly for each individual hospital, and then for all the hospitals combined.
Estimated MRSA burden (estimated prevalence), calculated as follows:
The number of patients (infected or colonised) with MRSA (previously diagnosed) X 10,000
Occupied bed days
Estimated MRSA acquisition (estimated incidence), calculated as follows:
The number of patients (infected or colonised) with newly-acquired, healthcare-related MRSA X 10,000
Occupied bed days
MRSA morbidity calculated as follows:
The number of newly-acquired MRSA infections X 10,000
Occupied bed days
The incidence and morbidity rates are also stratified by ICU or non-ICU.
These rates, along with those for previous months, are incorporated into individual hospital reports, which allow readers to both assess changes over time within their own hospitals and to compare their own rates for the three surveillance indicators with those for all hospitals combined. The highly-automated production of these reports (by way of Excel 'Automation'4) helps to ensure that reports are delivered to contributors in a timely manner.
Top of page
Results
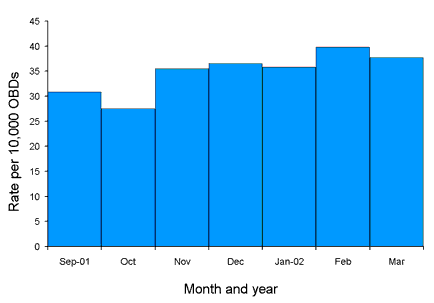
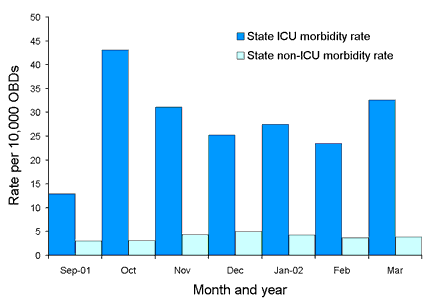
Using combined hospital data for the period 1 September 2001 to 31 March 2002, estimated burden rates range from 27.5 to 39.8 per 10,000 OBD per month (Figure 1). Estimated acquisition rates for the same period range from 28.2 to 69.0 per 10,000 OBD per month (for ICU) and from 6.3 to 10.1 per 10,000 OBD per month (non-ICU) (Figure 2). Morbidity rates range from 12.9 to 43.1 per 10,000 OBD per month (ICU) and from 3.0 to 5.0 per 10,000 OBD per month (non-ICU) (Figure 3).
Figure 1. State-wide estimated methicillin-resistant Staphylococcus aureus burden rates for the period 1 September 2001 to 31 March 2002
Top of page
Figure 2. State-wide estimated methicillin-resistant Staphylococcus aureus acquisition rates for the period 1 September 2001 to 31 March 2002
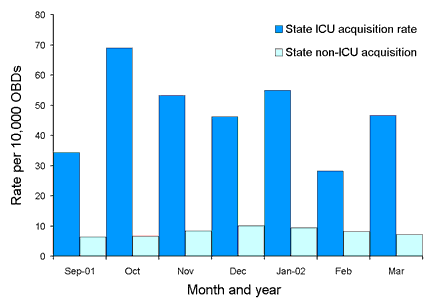
Figure 3. State-wide methicillin-resistant Staphylococcus aureus morbidity rates for the period 1 September 2001 to 31 March 2002
Top of page
Discussion
The release of the JETACAR report and the Commonwealth Government response to JETACAR heralded increased national concern over the public health threat posed by antibiotic-resistant bacteria. This is particularly the case for South Australia, where in 2002, media attention focused on several investigations into antibiotic-resistant bacteria in major public hospitals, helping to highlight the concern that rates of hospital-acquired infection due to antibiotic-resistant bacteria (including MRSA) may be increasing. Information from the Australian Group for Antimicrobial Resistance shows that the proportion of methicillin-resistant Staphylococcus aureus isolates in South Australia increased from 4 per cent in 1995 to 23 per cent in 1999 (Dr Peter Collignon, Chair of the Australian Group for Antimicrobial Resistance, personal communication).
Surveillance forms the cornerstone of control programs for all antibiotic-resistant organisms, including MRSA. The surveillance system described in this article has established baseline rates of MRSA acquisition and infection, and is enabling a better understanding of the epidemiology of this organism within South Australian public and private hospitals. We are now better positioned to both monitor the effects of new control measures, such as the use of new hand-hygiene products, or the use of antibacterial coated intravascular catheters, and to deal with new challenges, such as the introduction of new epidemic clones. Data collected will enable us to determine the common antibiotic resistance patterns in hospital-acquired MRSA in South Australia. This analysis has not yet been performed.
With regards to the reports produced by this surveillance system, the advice given by the SADHS to hospital staff is to focus primarily on improving their own rates over time. While there is a place for comparison with state-wide rates, at least in establishing whether or not a hospital's baseline rates are high, hospital staff are urged to do so with great caution. One factor affecting comparability is that South Australian hospitals vary with respect to factors known to influence rates of MRSA acquisition, such as the casemix and the burden of MRSA within the hospital. We are currently working with the contributing hospitals to develop a measure of relative casemix complexity, with the view to possibly adjusting rates according to this measure.
In order to generate high-quality information from this system, it is important to ensure that the contributing hospitals all implement the same surveillance methodology. The SADHS has confirmed that surveillance case definitions and MRSA screening policies (such as screening transfers from other healthcare institutions) are effectively the same for all the contributing hospitals; however, it is important to ensure that hospitals continue to comply with these policies over time.
Involvement in the South Australian MRSA surveillance system is voluntary. This decision was based on the view that mandatory data collection (particularly if attached to punitive consequences) may lead to under-reporting. The SADHS has implemented several strategies in order to encourage hospitals to contribute monthly data. Firstly, regular meetings are convened involving both SADHS staff and hospital infection control staff; these have helped to establish a collaborative relationship with contributing hospitals. Secondly, the system has been designed to ensure that information generated relating to individual hospitals remains confidential. Thirdly, contributors receive feedback in a timely manner, in part due to the high degree of automation achieved in the generation of reports.
Another strength of the South Australian MRSA surveillance system is the inclusion of private hospitals. These hospitals account for a large percentage (32%) of the total number of beds across all the contributing hospitals, and are undertaking increasingly complex procedures. Furthermore, the movement of patients and staff between public and private hospitals in South Australia is such that it makes little epidemiological sense to examine either system in isolation.
In conclusion, this recently-established enhanced surveillance system has improved our understanding of the epidemiology of hospital-acquired MRSA infections in South Australia. Our plan is now to develop similar systems in South Australia to study other resistant organisms, and to look at ways of relating resistance rates to rates of antibiotic usage.
Top of page
Acknowledgements
We would like to thank the following people for their assistance; members of the South Australian Nosocomial Infection Taskforce and the 16 contributing public and private hospitals for assisting with the development of the surveillance system, Mr Chris Horwood for developing and managing the database and assisting with other aspects of the project and Ms Caroline Gale for assisting with data collection.
Top of page
References
1. World Health Organization. World Health Organization consultation on global principles for the containment of antimicrobial resistance due to antimicrobial use in livestock, 5-6 June 2000, Geneva, Switzerland. Available from: http://www.who.int/emc/diseases/zoo/drafting.html
2. Commonwealth Department of Health and Aged Care. The Commonwealth Government Response to the Report of the Joint Expert Technical Advisory Committee on Antibiotic Resistance (JETACAR). Commonwealth Department of Health and Aged Care, Commonwealth Department of Agriculture, Fisheries and Forestry-Australia, Canberra 2000.
3. Australian Infection Control Association National Advisory Board. Draft surveillance indicator definitions: multi-resistant organisms. Australian Infection Control 2001;6:136-139.
4. McFedries P. Visual basic for applications unleashed. SAMS publishing; Indianapolis, 1997:1000.
Top of page
Author affiliations
1. Head, Infection Control Service, Communicable Disease Control Branch, South Australian Department of Human Services, Adelaide, South Australia
2. Infection Control Practitioner, Infection Control Service, Communicable Disease Control Branch, South Australian Department of Human Services, Adelaide, South Australia
Corresponding author: Dr Celia Cooper, Infection Control Service, Communicable Disease Control Branch, South Australian Department of Human Services, 162 Grenfell Street, Adelaide SA 5000. Telephone: +61 8 226 7177. Facsimile: +61 8 226 7187. Email: cdcb@dhs.sa.gov.au
This article was published in Communicable Diseases Intelligence Volume 27 Suppl, May 2003.
