The SENTRY Antimicrobial Surveillance Program was initiated in January 1997 and was designed to monitor the predominant pathogens and antimicrobial resistance for both nosocomial and community-acquired infections globally by using validated, reference-quality identification and susceptibility testing methods performed in a central laboratory. Consecutive bacterial or fungal isolates, deemed clinically significant by local criteria, are forwarded to the local reference laboratory from various study objectives. The major objectives include blood stream infections, community-acquired respiratory tract infections (Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis), pneumonias in hospitalised patients, skin and soft tissue infections, and urinary tract isolates from hospitalised patients. In 2001, special objectives were introduced to examine gastroenteritis pathogens and ▀-haemolytic streptococcal isolates. Over 22 nations participate in SENTRY surveillance globally. The Women's and Children's Hospital, Adelaide has been the reference centre for the Asia-Pacific region and South Africa since 1998, and three other Australian institutions, from Brisbane, Perth, and Adelaide, are part of the global network. All isolates received from our region are tested against up to 29 antimicrobial agents using custom-made broth microdilution panels. The data generated from SENTRY allows Australia to compare our antimicrobial resistance patterns and trends with our regional neighbours. Commun Dis Intell 2003;27 Suppl:S61-S66.
Top of page
Introduction
Surveillance programs are necessary to identify changes in the spectrum of microbial pathogens causing serious infection and to monitor trends in antimicrobial resistance patterns in nosocomial and community-acquired infections.1,2 The information gleaned from surveillance efforts is integral to the design of empirical approaches to the therapy of serious infection and also to defining appropriate control measures for antimicrobial-resistant pathogens.1 Such information has been provided in recent years by programs such as the National Nosocomial Infection Surveillance (NNIS) system,3 the Surveillance and Control of Pathogens of Epidemiologic Importance (SCOPE) program, and the Intensive Care Antimicrobial Resistance Epidemiology (ICARE) project.4 Australia currently has no formal mechanism for national antimicrobial resistance surveillance. The National Antimicrobial Resistance Surveillance Program (NARSP) was set up in 19911 but funding and contribution has been intermittent. Other surveillance is also being conducted on targeted pathogens by the Australian Group on Antimicrobial Resistance. These programs have been limited by their focus on nosocomial infections (NNIS and SCOPE) and by the lack of validated identification and antimicrobial susceptibility testing performed in a central laboratory (NNIS, ICARE and NARSP).
The SENTRY Antimicrobial Surveillance Program was initiated in January 1997 and was designed to monitor the predominant pathogens and antimicrobial resistance for both nosocomial and community-acquired infections globally by using reference quality identification and susceptibility testing methods performed in a central laboratory.
Top of page
Methods
Consecutive bacterial or fungal isolates, deemed clinically significant by local criteria, are forwarded to the local reference laboratory from five study objectives (Table 1). SENTRY sentinel sites for the Asia-Pacific and South Africa region (APAC) are shown in Table 2. The Women's and Children's Hospital, Adelaide has been the reference centre for the SENTRY-APAC since 1998. Recent publications from the SENTRY-APAC group have appeared elsewhere.5,6,7,8,9,10,11,12 All isolates were tested against more than 25 antimicrobials by the broth microdilution method using commercially prepared trays (TREK ™ Diagnostic Systems Limited, United Kingdom), according to National Committee for Clinical Laboratory Standards (NCCLS).13 Breakpoints for resistance were those recommended by the NCCLS.14 During the first four years, over 21,500 isolates from our region were tested.
Table 1. Major objectives under SENTRY surveillance, 1998 to 2001
Objective |
Infections and organisms |
| A |
Blood stream infections (bacteria and yeast) |
| B |
Community-acquired respiratory tract infections
(Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis) |
| C |
Pneumonias in hospitalised patients |
| D |
Skin and soft tissue infections from hospitalised patients |
| E |
Urinary tract infections in hospitalised patients |
| Special objectives (2001) |
| G |
Pathogens causing gastroenteritis |
| H |
ß-haemolytic Streptococci |
| I |
Pathogens from infected intensive care patients |
| NM |
Neisseria meningitidis |
Top of page
Table 2. SENTRY sentinel sites (Asia-Pacific Region and South
Africa)
Country |
Site location |
| Australia |
Princess Alexandra Hospital, Brisbane; Royal Perth Hospital,
Perth; Royal Adelaide Hospital, Adelaide; Women's and Children's Hospital,
Adelaide |
| Mainland China |
Beijing Medical University, Beijing; First Municipal
Peoples Hospital of Guangzhou, Guangzhou; Guangzhou Medical College First
Affiliated Hospital, Guangzhou |
| Hong Kong China |
Queen Mary Hospital, Hong Kong |
| Japan |
Kitasato University Hospital, Kitasato; Nagasaki University
Hospital, Nagasaki; Teikyo University Hospital, Tokyo |
| Philippines |
Mataki Medical Centre, Manila |
| Singapore |
Singapore General Hospital (till 1999); Tan Tock Seng
Hospital (since 2000) |
| Taiwan |
Chang Gung Memorial Hospital, Taoyuan; National Taiwan
University Hospital, Taipei; Veterans General Hospital, Taipei |
| South Africa |
Drs du Buisson, Bruinette and Partners, Johannesburg |
Top of page
Results
A snapshot of some key findings from our region is as follows:
Oxacillin-resistant Staphylococcus aureus
Oxacillin-resistant Staphylococcus aureus (ORSA) comprised more that 44 per cent of all S. aureus isolates from 17 of the 19 institutions participating since 1998. There is considerable regional variation in the prevalence of ORSA in our region. The prevalence of ORSA from blood stream infections from isolates collected from 1998 to 2001 is shown in Figure 1. Only one glycopeptide-intermediate (vancomycin MIC 8 mg/L) strain, from Hong Kong, has been detected to date.
Figure 1. Oxacillin-resistant Staphylococcus aureus blood stream infections, 1998 to 2001
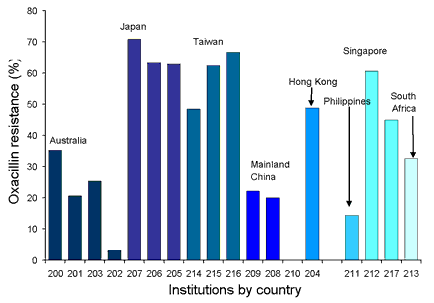
Top of page
Fluoroquinolone resistance
There were significant levels of fluoroquinolone resistance. The one exception is Australia where quinolone use in the community has been quite restricted. In the other countries there are high levels of resistance demonstrable in Enterobacteriaceae, non-fermentative Gram-negatives and oxacillin-resistant S. aureus. For some countries, the prevalence of ciprofloxacin resistance in Enterobacteriaceae is amongst the highest in the world. The prevalence of ciprofloxacin non-susceptible (MIC >1 mg/L) Escherichia coli is shown in Figure 2.
Figure 2. Prevalence of ciprofloxacin non-susceptible Escherichia coli, 1998 to 2001
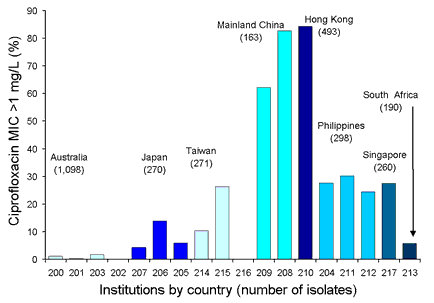
Top of page
Respiratory tract pathogens
Our region has some of the highest rates of penicillin resistance in Streptococcus pneumoniae in the world. Resistance was more prevalent in isolates from patients with respiratory tract infections (Figure 3) than those with blood stream infections (Figure 4). High-level resistance (MIC > 1 mg/L) was common in all participating countries except the Philippines, where S. pneumoniae were rarely recovered.
Figure 3. Respiratory tract isolates for penicillin resistant Streptococcus pneumoniae, 1998 to 2001
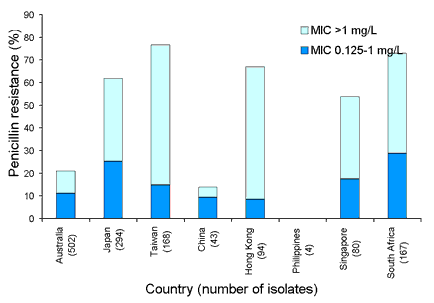
Figure 4. Penicillin resistant Streptococcus pneumoniae, blood stream isolates, 1998 to 2001
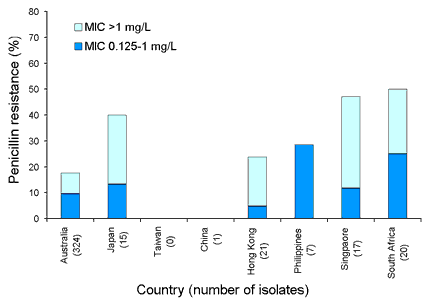
Top of page
Twenty-eight S. pneumoniae isolates, 15 of 130 (11.5%) from Hong Kong, 8 of 215 (3.7%) from one institution in Australia, 4 of 201 (2%) from two institutions in Japan and one from China, demonstrated high level resistance to fluoroquinolones (defined as an MIC > 32 mg/L). All isolates were resistant to all the recent agents (gatifloxacin, trovofloxacin, grepafloxacin, ofloxacin, levofloxacin).
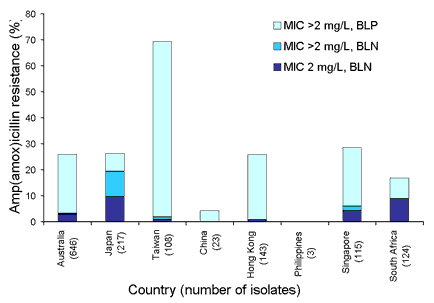
Of interest is the increasing incidence of ampicillin-resistant ▀-lactamase negative Haemophilus influenzae (BLNAR) (Figure 5). In Japan, these isolates account for nearly 10 per cent of all respiratory isolates and 58 per cent of those strains that were ampicillin resistant. BLNAR have now been detected in Australia. Alarming levels of resistance are seen in Taiwan, with many of the isolates being multi-drug resistant.
Figure 5. Respiratory tract isolates for amp(amox)icillin resistant Haemophilus influenzae, 1998 to 2001
Top of page
Extended-spectrum ▀-lactamases
Australia had the lowest incidence of extended-spectrum ▀-lactamase (ESBL) producing K. pneumoniae and E. coli in the APAC region. Over 30 per cent of K. pneumoniae isolates from patients with blood stream infection from Mainland China, Philippines, Singapore and South Africa were confirmed as having an ESBL (Table 3). Mainland China and Hong Kong China also had significant numbers of ESBL-producing E. coli (24% and 13% respectively). Although multi-resistance was common, no carbapenem resistance was detected. More than one third of isolates were also resistant to cefoxitin, consistent with them also possessing Class 1 cephalosporinases. Nearly half of these isolates were from a single institution, and in two species, (E. coli and K. pneumoniae) suggesting both clonal dissemination and plasmid transfer of resistance. Over 75 per cent of cefoxitin-resistant ESBL isolates were found in three countries (Philippines, China and Hong Kong).15
Table 3. Variation in rates of extended spectrum ▀-lactamase production among Escherichia coli and Klebsiella pneumoniae blood isolates by region at SENTRY-APAC centres, 1998 to 1999
| Country |
E. coli |
K. pneumoniae |
| |
n |
%* |
n |
%* |
| Australia |
286 |
0.0 |
51 |
2.0 |
| Hong Kong |
138 |
13.0 |
38 |
7.9 |
| Japan |
74 |
1.4 |
44 |
15.9 |
| Mainland China |
75 |
24.0 |
23 |
65.2 |
| Philippines |
65 |
6.2 |
44 |
31.8 |
| Singapore |
50 |
4.0 |
39 |
41.0 |
| South Africa |
30 |
3.3 |
22 |
45.5 |
| Taiwan |
29 |
13.8 |
56 |
5.4 |
| Total |
774 |
6.1 |
290 |
24.1 |
Top of page
Discussion
The global nature of antimicrobial resistance requires that resistance data from both developed and developing countries be collected. New resistance phenotypes remain on the rise while multidrug-resistant pathogens continue to spread. The data generated from SENTRY allows Australia to compare our antimicrobial resistance patterns and trends with our regional neighbours, who have some of the highest reported resistances in the world. Ongoing surveillance such as obtained by the SENTRY program is essential to monitor the emerging resistances, and also to evaluate new antimicrobial agents as they become available.
Top of page
Acknowledgement
The SENTRY Program is funded by an educational grant from the Bristol-Myers Squibb Pharmaceutical company.
Top of page
References
1. Turnidge J, Bell J, Collignon P. The battle against antibiotic resistance. Microbiology Australia 1996;17:27-31.
2. Jones RN. The emergent needs for basic research, education, and surveillance of antimicrobial resistance. Problems facing the report from the American Society for Microbiology Task Force on Antibiotic Resistance. Diagn Microbiol Infect Dis 1996;25:153-161.
3. Emori TG, Culver DH, Horan TC, Jarvis WR, White JW, Olson DR, et al. National nosocomial infections surveillance system (NNIS): description of surveillance methods. Am J Infect Control 1991;19:19-35.
4. Archibald L, Phillips L, Monnet D, McGowan JE, Tenover F, Gaynes R. Antimicrobial resistance in isolates from inpatients and outpatients in the United States: increasing importance of the intensive care unit. Clin Infect Dis 1997;24:211-215.
5. Diekema DJ, Pfaller MA, Turnidge J, Verhoef J, Bell J, Fluit AC, et al. Genetic relatedness of multidrug-resistant, methicillin (oxacillin)-resistant Staphylococcus aureus bloodstream isolates from SENTRY antimicrobial resistance surveillance centers worldwide, 1998. Microb Drug Resis 2000;6:213-221.
6. Turnidge JD, Bell JM. Methicillin-resistant Staphylococcus aureus evolution in Australia over 35 years. Microb Drug Resis 2000;6:223-229.
7. Diekema DJ, Pfaller MA, Schmitz FJ, Smayevski J, Bell JM, Jones RN, et al. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe and the western Pacific for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin Infect Dis 2001;32 Suppl 2:S114-S132.
8. Bell JM, Turnidge JD, SENTRY APAC. High prevalence of oxacillin-resistant Staphylococcus aureus isolates from hospitalised patients in Asia-Pacific and South Africa: Results from the SENTRY Antimicrobial Surveillance Program, 1998-1999. Antimicrob Agents Chemother 2002;46:879-881.
9. Bell JM, Turnidge JD, Gales AC, Pfaller MA, Jones RN, the SENTRY APAC Study Group. Prevalence of extended spectrum beta-lactamase (ESBL)-producing clinical isolates in the Asia-Pacific region and South Africa: regional results from SENTRY Antimicrobial Surveillance Program, (1998-99). Diagn Microbiol Infect Dis 2002;42:193-198.
10. Bell JM, Turnidge JD, Jones RN, the SENTRY Regional Participants Group. Antimicrobial resistance trends in community-acquired respiratory tract pathogens in the Western Pacific Region and South Africa: report from the SENTRY Antimicrobial Surveillance Program, (1998-1999) including an in vitro evaluation of BMS284756. Int J Antimicrob Agents 2002;19:125-132.
11. Bell JM, Turnidge JD, Pfaller MA, Jones RN. In vitro assessment of gatifloxacin spectrum and potency tested against Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae isolates from the Asia-Pacific component of the SENTRY Antimicrobial Surveillance Program (1998-1999). Diagn Microbiol Infect Dis 2002;43:315-318.
12. Turnidge JD, Bell JM, Biedenbach DJ, Jones RN. Pathogen occurrence and antimicrobial resistance trends among urinary tract infection isolates in the Asia-Western Pacific Region: Report from the SENTRY Antimicrobial Surveillance Program, 1998-1999. Int J Antimicrob Agents 2002;20:10-17.
13. National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved Standard-Fifth Edition [M7-A5]. National Committee for Clinical Laboratory Standards, Wayne, Pennsylvania, 2000.
14. National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing; Twelfth informational supplement [M100-S12]. National Committee for Clinical Laboratory Standards, Wayne, Pennsylvania, 2002.
15. Bell JM, Turnidge JD, the SENTRY Western Pacific Plus Participants. Paper C2-1477 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, 26-29 September 1999, San Francisco.
Top of page
Author affiliations
1. Microbiology and Infectious Diseases, Women's and Children's Hospital, Adelaide, South Australia
2. Director of Microbiology and Infectious Diseases, Women's and Children's Hospital, Microbiology Department, North Adelaide, South Australia
Corresponding author: Ms Jan Bell, Microbiology and Infectious Diseases, Women's and Children's Hospital, Adelaide SA 5006. Telephone: +61 8 8161 6359. Facsimile: +61 8 8161 6051. Email: bellj@mail.wch.sa.gov.au
This article was published in Communicable Diseases Intelligence Volume 27 Suppl, May 2003.
