A randomised controlled trial involving 54 general practitioners (GPs) was conducted in Canberra, Australian Capital Territory from September 1997 to November 1999. In the first year of the study, 24 GPs, who constituted the active arm of the intervention group, were involved in the consideration of evidence and the development and implementation of a set of clinical guidelines for the treatment of acute respiratory infections. These guidelines were then endorsed in a meeting together with specialist colleagues. In the second year of the study the group of GPs who had been acting as controls, received a moderate intervention consisting of a brief educational event and distribution of the locally developed guidelines. We obtained data from January 1997 to December 1999 from the Health Insurance Commission on prescribing rates for 40 of the doctors in the study. The rate of prescribing was calculated as the number of antibiotic prescriptions per 100 Medicare services. The average yearly prescribing decreased significantly in the intensive intervention group and increased in the moderate intervention group (p=0.026). A mixed effects longitudinal time series model was fitted to the data to account for seasonal variation of antibiotic prescribing and trends over time. The intensive intervention group significantly reduced their antibiotic prescribing over time compared to the moderate intervention group (p<0.001). This study has shown that an intensive intervention in which general practitioners were actively engaged in development and consideration of the evidence base for the guidelines resulted in a significant fall in general antibiotic prescribing. Commun Dis Intell 2003;27 Suppl:S32-S38.
Top of page
Introduction
Acute respiratory infections (ARI) are a very significant part of the workload of general practitioners (GPs). Antibiotics are frequently used to treat these generally self-limiting infections. For different types of respiratory infections, the antibiotic prescribing rate in general practice ranges from 50 to 90 per cent.1
Numerous studies have demonstrated that antibiotics offer at best a modest benefit for most acute respiratory infections. Antibiotics are not indicated for simple upper respiratory tract infections.2,3,4 However, upper respiratory tract infection is often associated with bronchitis, pharyngitis, otitis media or sinusitis. These conditions can sometimes benefit from antibiotic treatment in a subset of patients.5,6,7,8,9,10,11,12,13 The difficulty for the clinician is to distinguish, at the time of the consultation, which subset of patients would benefit from antibiotics and which would not. The signs and symptoms of these illnesses may not clearly differentiate between these groups of patients. A watchful approach may often be justified,14,15 but the pressures of modern practice are such that clinicians currently tend to overuse these drugs.
In light of growing antibiotic resistance and the modesty of the clinical benefit16 calls have been made for a more judicious use of antibiotics for the treatment of acute respiratory infections.17 Yet the task of altering established practices of antibiotic use remains an unmet challenge.18
This paper reports on the effect on general prescribing practices of a randomised controlled trial that tested two different approaches to the implementation of clinical guidelines for antibiotic use in management of childhood respiratory infections.
Top of page
Methods
We undertook a two year randomised controlled trial to explore the effects of a unique method of clinical practice guideline development and implementation on antibiotic use for acute respiratory infections in young children. The recruitment of participants and methods of guideline development are described fully elsewhere.19 Briefly, 54 GPs from practices in Canberra, Australian Capital Territory (one GP per practice) were recruited into the study beginning in September 1997. GPs were randomly allocated into an intensive intervention group or a moderate intervention group. The intensive intervention consisted of a series of focus groups with parents of young children and workshops with the study GPs (beginning in the first quarter of 1998). Clinical practice guidelines for the management of childhood ARI were collaboratively developed in these focus groups and workshops. Evidence for antibiotic treatment of ARI from Cochrane reviews and other studies was explored and examined in light of the experiences and expectations of GPs and consumers in the management of these illnesses. Barriers to the judicious use of antibiotics for ARI were identified and discussed and an implementation package developed which addressed these barriers to change. This package consisted of:
- Guidelines for GPs: The guidelines were entitled Principles of Practice and stated the principles of management of ARI that the GPs had agreed upon during the development process. The guidelines were flexible and allowed for the realities of general practice and patient preference. They incorporated the evidence and were tailored to GP and patient concerns to allow for ownership of the guidelines.
- Information sheets on otitis media and sore throat: Copies of these sheets were given to GPs to distribute to patients. The sheets were developed by Dr Chris Del Mar, one of the authors for the Cochrane review on antibiotic treatment of otitis media11 and sore throat8 and kindly given to us for use in this study. The information sheets allowed for the education of patients in a time efficient manner.
- ARI management prescription pad: These prescription-like pads allowed the GP to tick a series of boxes that explained the diagnosis, recommended symptomatic treatment, and caution on the warning signs if the patient did not have any improvement. The prescription pads also contributed to the education of the patient by providing clear advice on self-management.
- Poster: We developed a colourful poster to be displayed in the GPs surgery that advocated the judicious use of antibiotics. The poster helped to market the new way of practice.
Top of page
The implementation package was distributed to the intensive intervention group in the first year of the study shortly after the last workshop session (April 1998). The moderate intervention group acted as a control for the ensuing year.
In the second quarter of 1999, the moderate intervention group was provided with the locally developed guidelines and implementation package at an evening educational event. At this time the intensive intervention group received prescribing feedback and reinforcement of the guideline principles at a follow-up evening group meeting. Thus the study tested the comparative effects over time of two levels of intervention, an intensive intervention and a moderate intervention. Although not part of this analysis, the study also provided guidelines to parents and education through newsletters.
GP antibiotic prescribing was monitored using Health Insurance Commission (HIC) data obtained with GP's permission from 40 of the 49 GPs still active in the study by the end of 1999, 20 from each intervention group. The total number of antibiotic prescriptions filled for patients eligible for Pharmaceutical Benefits Scheme subsidy and total number of Medicare services provided by the GPs in the two groups were calculated by calendar quarter from 1997 through 1999 and by calendar year. The variable of interest was the number of subsidised antibiotic prescriptions per 100 Medicare services.
A mean rate of prescribing was calculated for each year of the study and the change in prescribing from the baseline year (1997) to the intervention years of 1998 and 1999 were compared using t-tests. To account for the seasonal variation in antibiotic prescribing, detail which is lost using a yearly mean rate, a mixed effects longitudinal time series model was fitted to the HIC data using Stata version 7 software.20 The model incorporated a random effect clustered by GP and fixed effects of intervention group and sine and cosine terms to account for the seasonal variation of antibiotic prescribing. In addition, a linear term in time was added to measure trend within the intervention groups.
Top of page
Results
Antibiotic prescribing followed a cyclical seasonal pattern. Prescribing was lowest in the summer months (first quarter) and highest in the winter months (third quarter). Table 1 details the number of antibiotic prescriptions per 100 Medicare services by intervention group and quarter.
Table 1. Mean number of antibiotic prescriptions per 100 Medicare services by intervention group and yearly quarter
Quarter* |
Group |
Mean ± SD |
95% CI |
| Q1 –1997 |
IIG
MIG |
6.67 ± 2.7
5.68 ± 1.7 |
5.4, 7.9
4.8, 6.5 |
| Q2 –1997 |
IIG
MIG |
7.66 ± 2.6
7.61 ± 3.3 |
6.4, 8.9
6.1, 9.1 |
| Q3 –1997 |
IIG
MIG |
8.73 ± 2.8
8.39 ± 2.5 |
7.4, 10.1
7.2, 9.6 |
| Q4 –1997 |
IIG
MIG |
7.01 ± 2.4
6.96 ± 1.9 |
5.9, 8.1
6.1, 7.8 |
| Q1 –1998 |
IIG
MIG |
5.92 ± 2.1
5.82 ± 1.7 |
4.9, 6.9
5.0, 6.6 |
| Q2 –1998 |
IIG
MIG |
6.16 ± 2.3
7.14 ± 2.2 |
5.1, 7.2
6.1, 8.2 |
| Q3 –1998 |
IIG
MIG |
8.00 ± 3.3
7.92 ± 2.1 |
6.5, 9.5
6.9, 8.9 |
| Q4 –1998 |
IIG
MIG |
7.53 ± 2.8
7.61 ± 2.4 |
6.2, 8.8
6.5, 8.7 |
| Q1 –1999 |
IIG
MIG |
6.43 ± 2.4
6.33 ± 2.4 |
5.3, 7.6
5.2, 7.4 |
| Q2 –1999 |
IIG
MIG |
6.47 ± 2.3
7.14 ± 2.5 |
5.4, 7.5
6.0, 8.3 |
| Q3 –1999 |
IIG
MIG |
7.16 ± 3.5
8.60 ± 2.5 |
5.5, 8.8
7.4, 9.8 |
| Q4 –1999 |
IIG
MIG |
6.90 ± 3.1
8.00 ± 2.6 |
5.5, 8.3
6.8, 9.2 |
Antibiotic prescribing during the baseline year of 1997 was not significantly
different between the intensive and moderate intervention groups as shown in
Table 2. Over the course of the study the mean rate of prescribing for the
intensive intervention group decreased by –0.78 prescriptions per 100
Medicare services whereas the mean rate of prescribing increased by 0.35 prescriptions
in the moderate intervention group, a difference of –1.13, 95 per cent
CI (-2.1, –0.1) and p=0.026 (Table 2).
Top of page
Table 2. Baseline level of antibiotic prescribing and mean
yearly change
Group |
Baseline prescribing in 1997
(number of prescriptions per 100 Medicare services) |
Mean change* from 1997 to 1998 |
Mean change from 1997 to 1999 |
| Intensive intervention group |
7.52 ± 2.4 |
–0.62 ± 1.3 |
–0.78 ± 1.3 |
| Moderate intervention group |
7.16 ± 2.2 |
–0.04 ± 0.8 |
0.35 ± 1.7 |
p value
95% confidence interval |
0.630
(–1.1, 1.8) |
0.100
(–1.3, 0.1) |
0.026
(–2.1, –0.1) |
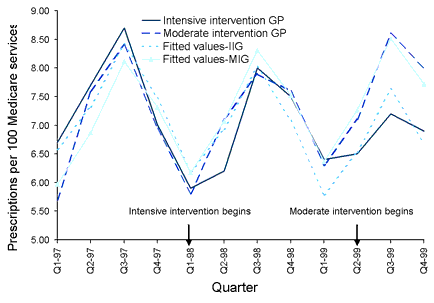
The seasonal pattern of prescribing was accounted for by a mixed effects longitudinal time series model fitted to the HIC data. There was a highly significant interaction between intervention group and time, p<0.001, whereby the intensive intervention group reduced their antibiotic prescribing over time compared to the moderate intervention group (▀ coefficient = -0.15, 95% CI -0.22, -0.07). The Figure shows the fitted curve of the model with the data for mean rates of prescribing by quarter for each intervention group.
Figure. Prescriptions per 100 Medicare services in groups of GPs before and after intensive or moderate interventions to revise prescribing practices for upper respiratory tract infections, means and fitted values
Top of page
Discussion
A detailed analysis of the randomised trial that involved 54 general practitioners and 502 of their child patients has been presented elsewhere.21 That report showed that GPs in the intensive intervention group changed their prescribing practices in relation to the study children. The analysis in the present paper was undertaken to test whether this change in prescribing behaviour, which was evident for children enrolled in the study, carried over into the overall practices of the doctors, including patients who were not enrolled in the very intense diary keeping study. This study has shown that a multi-faceted evidence-based approach to guideline development and implementation was effective in reducing general antibiotic prescribing.
The use of the Pharmaceutical Benefits Scheme and Medicare data involved some limitations but provided an objective measure of prescribing behaviour over time. The Health Insurance Commission will hold a record of a prescription only if the cost of the drug is higher than the patient contribution, approximately A$21.00 at the time of the study. However, prescriptions from pensioners and holders of concession cards is recorded. Thus the data capture for antibiotic prescriptions, which generally cost less than the patient contribution, was incomplete. This incomplete data capture, however, would have been equal for both intervention groups and not a source of bias. Furthermore, HIC data has been used previously to monitor antibiotic prescribing.28 Only 40 of the 49 GPs active at the end of the study gave written permission to obtain their prescribing information from the HIC. Those that did not comply, however, were equally distributed between the two groups.
Clinical practice guidelines have long been seen as a way to enhance best practice in health care. The National Health and Medical Research Council has published a series of handbooks detailing the main stages of clinical practice guideline development and implementation.22 However, observed change in studies of guideline development and implementation have often been modest.23,24,25 Single interventions frequently resulted in little or no change in behaviour but complex interventions using several methods had a better chance of producing change.26,27 Nevertheless, all make it clear that, as Oxman et al declare, 'there are no magic bullets'.25 There is no one method that will effectively change clinical behaviour.
Top of page
Grol and Grimshaw18 provide a general framework for integration of evidence with clinical practice. They advocate an evidence-based multi-faceted approach, the careful assessment of barriers to change, and tailoring interventions to specifically address the barriers. This general framework was used to guide the approach of guideline development and implementation described in this study. We first started with the evidence for the judicious use of antibiotics for ARI. Then the barriers to incorporating the evidence into practice for GPs and consumers were assessed through the process of guideline development using focus groups and workshops. The identification of these barriers have been presented previously.19 Discussing the evidence, identifying the barriers to incorporate the evidence into practice, openly exploring these issues with the GPs in a collaborative peer environment, and devising an implementation package to address these barriers contributed to the development of guidelines that were effective for changing practice in the intensive intervention group of GPs. Furthermore, ongoing contact was maintained with the intensive intervention group through feedback reports and reinforcement of guidelines. A moderate intervention of distribution of the locally developed guidelines and implementation package with a brief educational component was not sufficient to reduce antibiotic prescribing in the moderate intervention cohort of GPs.
Although labour intensive, the workshops, which were the main difference between the intensive and moderate interventions, were effective in changing clinical behaviour. The process of examining the evidence and discussing this with their colleagues during several sessions, with the support of academic GPs to clarify the evidence, was more influential in changing GP behaviour than distribution of the evidence-based guideline package with a brief educational event (moderate intervention).
Others have shown that passive dissemination of guidelines,23 unsolicited feedback reports,28 or didactic continuing medical education28 are generally ineffective in changing clinical behaviour. This study has shown that antibiotic prescribing can be significantly reduced using methods that discuss the evidence in a peer-supported environment. The need for effective methods to reduce antibiotic prescribing becomes more urgent in light of the results from companion research to this study that has shown antibiotic use in the community directly correlates with the level of antibiotic resistance.29 In Australia there may be a role for the Divisions of General Practice, in conjunction with academic institutions, to organise workshop series with GPs and focus groups with consumers for the open examination of evidence, experiences, and preferences to enhance the practice of evidence-based health care.
Top of page
Acknowledgements
This work was supported by a grant from the General Practice Evaluation Program of the General Practice Branch of the Commonwealth Department of Health and Ageing. We would also like to thank the Health Insurance Commission for providing the data for the evaluation and all the GPs who participated in the study.
Top of page
References
1. Bridges-Webb C, Britt H, Miles DA, Neary S, Charles J, Traynor V. Morbidity and treatment in general practice in Australia 1990-1991. Med J Aust 1992;157 Suppl:S1-S56.
2. Rosenstein N, Phillips WR, Gerber MA, Marcy SM, Schwartz B, Dowell SF. The common cold -principles of judicious use of antimicrobial agents. Pediatrics 1998;101 Suppl:S181-S184.
3. Mashford ML, Beaves M, Hogg G, Kucers A, Robinson G, Spicer JW, et al. Antibiotic Guidelines 9th edition. Victorian Medical Postgraduate Foundation Inc; Melbourne, 1996.
4. Arroll B, Kenealy T. Antibiotics versus placebo in the common cold (Cochrane review). In: The Cochrane Library. Oxford, Issue 1, 1999.
5. Becker L, Glazier R, McIsaac W, Smucny J. Antibiotics for acute bronchitis. Cochrane Database Syst Rev 1997;4.
6. Orr PH, Scherer K, Macdonald A, Moffatt ME. Randomized placebo-controlled trials of antibiotics for acute bronchitis: a critical review of the literature. J Fam Pract 1993;36:507-512.
7. O'Brien K, Dowell S, Schwartz B, Marcy S, Phillips W, Gerber M. Cough illness/bronchitis -principles of judicious use of antimicrobial agents. Pediatrics 1998;101 Suppl:S178-S181.
8. Del Mar C, Glasziou P. Antibiotics for the symptoms and complications of sore throat. In: The Cochrane Library. Oxford, 1996.
9. Zwart S, Sachs AP, Ruijs GJ, Gubbels JW, Hoes AW, de Melker RA. Penicillin for acute sore throat: randomised double blind trial of seven days versus three days treatment or placebo in adults. BMJ 2000;320:150-154.
10. Froom J, Culpepper L, Jacobs M, DeMelker RA, Green LA, van Buchem L, et al. Antimicrobials for acute otitis media? A review from the International Primary Care Network. BMJ 1997;315:98-102
11. Glasziou P, Hayem M, Del Mar C. Treatments for acute otitis media in children: antibiotic versus placebo. In: The Cochrane Library. Oxford, 1996.
12. O'Brien KL, Dowell SF, Schwartz B, Marcy SM, Phillips WR, Gerber MA. Acute sinusitis -principles of judicious use of antimicrobial agents. Pediatrics 1998;101 Suppl:S174-S177.
13. Winther B, Brofeldt S, Gronborg H, Mygind N, Pedersen M, Vejlsgaard R. Study of bacteria in the nasal cavity and nasopharynx during naturally acquired common colds. Acta Otolaryngol 1984;98:315-320.
Top of page
14. Little P, Gould C, Williamson I, Moore M, Warner G, Dunleavey J. Pragmatic randomised controlled trial of two prescribing strategies for childhood acute otitis media. BMJ 2001;322:336-342.
15. Little P, Gould C, Williamson I, Warner G, Gantley M, Kinmonth A. Reattendance and complications in a randomised trial of prescribing strategies for sore throat: the medicalising effect of prescribing antibiotics. BMJ 1997;315:350-352.
16. Turnidge JD, Bell JM, Collignon PJ. Rapidly emerging antimicrobial resistances in Streptococcus pneumoniae in Australia. Med J Aust 1999;170:152-155.
17. The Commonwealth Government Response to the Report of the Joint Expert Technical Advisory Committee on Antibiotic resistance (JETACAR). Commonwealth Department of Health and Aged Care, Commonwealth Department of Agriculture, Fisheries and Forestry-Australia, Canberra, 2000:39.
18. Grol R, Grimshaw J. Evidence-based implementation of evidence-based medicine. Jt Comm J Qual Improv 1999;25:503-513.
19. Wilson EJ, Nasrin D, Banwell C, Broom D, Douglas RM. Realities of practice. Engaging parents and GPs in developing clinical practice guidelines. Aust Fam Physician 2000;29:498-503.
20. Stata Statistical Software 6.0 version. College Station, TX: Stata Corporation, 1999.
21. Wilson E. Realities of practice: development and implementation of clinical practice guidelines for acute respiratory infections in young children [PhD thesis]. The Australian National University, 2002.
22. National Health and Medical Research Council. Series of handbooks on the main stages involved in the development of clinical practice guidelines. Biotex Canberra, 2000. Available from: http://www7.health.gov.au/nhmrc/publications/synopses/cp65syn.htm Accessed 2001.
23. Davis DA, Taylor-Vaisey A. Translating guidelines into practice. A systematic review of theoretic concepts, practical experience and research evidence in the adoption of clinical practice guidelines. CMAJ 1997;157:408-416.
24. Lomas J. Words without action? The production, dissemination, and impact of consensus recommendations. Annu Rev Public Health 1991;12:41-65.
25. Oxman A, Thomson M, Davis D, Haynes B. No magic bullets: a systematic review of 102 trials of interventions to improve professional practice. CMAJ 1995;153:1423-1431.
26. Bero LA, Grilli R, Grimshaw JM, Harvey E, Oxman AD, Thomson MA. Closing the gap between research and practice: reviews of interventions to promote implementation of research findings by health care professionals. In: Haines A, Donald A, editors. Getting Research Findings into Practice. London: BMJ Publishing, 1998:160.
27. Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care 2001;39 Suppl 2:II 46-II 54.
28. O'Connell DL, Henry D, Tomlins R. Randomised controlled trial of effect of feedback on general practitioners' prescribing in Australia. BMJ 1999;318:507-511.
29. Nasrin D, Collignon PJ, Roberts L, Wilson EJ, Pilotto LS, Douglas RM. Effect of beta lactam antibiotic use in children on pneumococcal resistance to penicillin: prospective cohort study. BMJ 2002;324:28-32.
Top of page
Author affiliations
1. PhD Scholar, National Centre for Epidemiology and Population Health, Australian National University, Canberra, Australian Capital Territory
2. Senior Fellow, National Centre for Epidemiology and Population Health, Australian National University, Canberra, Australian Capital Territory
3. Director, National Centre for Epidemiology and Population Health, Australian National University, Canberra, Australian Capital Territory
Corresponding author: Dr Eileen J Wilson, Epidemiologist, Defence Links Branch, Department of Veterans' Affairs, Canberra ACT 2602. Telephone: +61 2
6289 1110. Facsimile: +61 2 6289 6173. Email: eileen.wilson@dva.gov.au
This article was published in Communicable Diseases Intelligence Volume 27 Suppl, May 2003.
