The development of antibiotic utilisation monitoring and antibiotic guidelines in a 600-bed tertiary referral hospital is described. Antibiotic utilisation data were collected into a spreadsheet and increases in usage of antibiotics were investigated. Hospital-specific antibiotic guidelines were developed, promoted and reviewed. Adherence to the guidelines for restricted antimicrobials was monitored and prescribing outside the guidelines was discussed with the prescriber. The development of a 'pneumonia card' and guidelines for antibiotic use in surgical prophylaxis and wound infection are described and evidence for improved rates of appropriate prescribing are presented. A "Switch to Oral" campaign aimed at reducing intravenous antibiotic use is described. There is evidence of decreased nosocomial infections rates in the hospital as a result of these activities. Commun Dis Intell 2003;27 Suppl S13-S18.
Top of page
Introduction
The John Hunter Hospital, which is a 600 bed tertiary referral centre, has an antimicrobial working party (AWP) comprising representatives from pharmacy, microbiology and infectious diseases fields of knowledge. This group, which reports to the Hunter Area Health Service Quality Use of Medicines Committee, is responsible for the monitoring of antibiotic usage and the development, implementation and evaluation of guidelines for the appropriate use of antimicrobials. Activities include the development and promotion of a restricted antimicrobial policy including specific guidelines for the management of pneumonia, surgical prophylaxis and wound infection and approved indications for antibiotics identified as requiring restrictions. The Hunter Area Health Service adopted this restricted anti-infective policy in 2001. Support for the implementation of this policy at smaller hospitals within the area is provided by members of the AWP.
Top of page
Monitoring
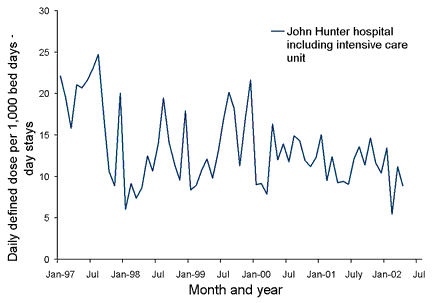
Antibiotic utilisation is monitored using World Health Organization Defined Daily Dose measures per 1,000 bed days and trends are evaluated at the end of each month. Increases in usage are investigated and interventions developed as necessary. A template of the spreadsheet is available on request by sending an email to jmacdonald@hunter.health.nsw.gov.au. Data can be entered into the spreadsheet to produce graphs such as the example shown (Figure).
Figure. Third generation cephalosporin usage at John Hunter Hospital, January 1997 to April 2002, example of graphs which can be produced by the John Hunter Hospital spreadsheet
Drug Utilisation Evaluation (DUE) is a structured ongoing system for monitoring drug use through comparisons with existing standards and guidelines. DUE is a cycle of audit, educational intervention and review, which aims to measure prescribing of target drugs and provide feedback to prescribers. The Greater Newcastle Sector has a dedicated team who conducts DUE projects throughout the region.
Top of page
Promotion of guidelines
Restricted antibiotic guidelines
Hospital specific guidelines have been developed, in line with the Therapeutic Guidelines: Antibiotic,1 taking into consideration input from relevant clinical units, published evidence (where available) and local resistance data. The main aims of the guidelines are to promote appropriate use of broad-spectrum antimicrobials in order to limit the development and spread of antimicrobial resistance, and to ensure appropriate use of specific agents. These guidelines are available on the hospital intranet, the VAX network system and in hard copy on all wards. The guidelines are reviewed regularly according to clinical needs and formulary changes. Active promotion of the guidelines is undertaken at medical staff orientation and during a 2 week intensive audit and intervention period four times per year, corresponding with the registrar rotation. Education and feedback to specific groups is undertaken as required. Clinical pharmacists consult with prescribing doctors regarding their choice of antibiotic and the Infectious Diseases (ID) service is available around the clock to review requests outside of the guidelines.
Adherence to the guidelines for prescribing of restricted antimicrobials is monitored rather than policed, and feedback is intended to be educational. Clinical pharmacists review all restricted antimicrobials dispensed (capturing about 85% of all courses) daily, and note non-compliance with the guidelines. If the clinical pharmacist considers it necessary, the prescribing team is contacted and the issue is discussed. Reference is made to the hospital guidelines and the Therapeutic Guidelines: Antibiotic. If, however, the clinical team is reluctant to change their anti-infective choice then a consultation with the ID service can occur. Clinical pharmacists undertake this level of intervention as part of their ward-based service. Having the support of the ID team allows the pharmacists to avoid a proscriptive approach. Members of the AWP meet weekly and review the use of restricted antimicrobials outside the guidelines, and feedback to individual medical officers is undertaken where necessary. Specific education and feedback to a particular clinical unit or group of prescribers has also occurred as required. Over time, this united, educative approach has reinforced the importance of appropriate anti-infective prescribing and improved discussion surrounding prescribing choices.
During the quarterly audit and intervention period, all courses of restricted antimicrobials are reviewed, including those non-dispensed, i.e., from imprest stocks. Non-adherence to the guidelines is addressed by members of the AWP within 24 hours. The ID consultant, after reviewing the indication for antibiotic therapy, contacts the prescribing team, suggests alternative therapy if indicated and explains the guidelines. The emphasis is on education. Data collected on the use of restricted antibiotics between 2 and 15 February 2001 is presented in the Table. Of the 22 courses of antibiotics not approved, 7 (32%) were for (suspected or proven) intra-abdominal sepsis, 6 (27%) for respiratory tract infection, 3 (14%) for urinary tract infection, and 6 (27%) for other reasons (including surgical prophylaxis, cellulitis, surgical wound infection). The main reasons for non-concordance with the hospital guidelines were either the availability of less broad-spectrum antimicrobials that would provide adequate cover for the condition or organism concerned, or that antibiotic therapy was not considered necessary by the ID team.
Table 1. Use of restricted antibiotics, 2 January to 15 February 2001
Division |
Courses of restricted antibiotics |
Approved
(% for Division) |
Not approved
(% for Division |
Approved but used outside guidelines |
| Medicine |
51 (39.5%) |
42 (82.3%) |
9 (17.7%) |
13 (25.5%) |
| Obstetrics and gynaecology |
1 (0.7%) |
0 |
1 |
0 |
| Paediatrics |
24 (18.6%) |
21 (87.5%) |
3 (12.5%) |
6 (25.0%) |
| Surgery |
53 (41.1%) |
41 (77.4%) |
9 (17.0%) |
11 (20.8%) |
Pneumonia guidelines
The Pneumonia Guidelines were developed by the AWP in September 1998
in conjunction with the evidence-based review of pneumonia management that
took place for the Therapeutic Guidelines: Antibiotic, edition 10.2 Consultation
was undertaken with the Respiratory Medicine, Accident and Emergency and Intensive
Care units at John Hunter Hospital, and the guidelines were updated and ratified
in May 2001. The pneumonia card (Box), was developed as a tool for ready reference
by clinical staff. This card is small enough to attach to the hospital identification
tag and has been distributed widely amongst medical officers. Every junior
medical officer is given a card with education at orientation. Active promotion
of the card is undertaken, including intensive promotion each year leading
into the winter months. Anecdotal feedback and requests for cards from clinicians
indicate that this is a worthwhile method of information dissemination.
Box. The pneumonia card, (front and back)
Hospital-acquired pneumonia (HAP)
Empiric antibotic therapy
See JCLIN(VAX) or the HAHS intranet for dosages, advice on investigation
and other alternatives for ß-lactam allergic patients
Mild/moderate
No risk factors: penicillin G + gentamicin (IV) OR amoxycillin/clavulanate(oral)
Witnessed aspiration: pen G + gentamicin + metronidazole
Head injury, coma, diabetes, dialysis: pen G + gentamicin + di/flucloxacillin
OR if MRSA proven: vancomycin + gentamicin
ICU cases or Severe
Onset less than 5 days post admission & no risk factors:
penicillin G + gentamicin. If severe, add erythromycin as per severe
CAP
Other cases:
gentamicin + ticarcillin/clavulanate (gent + cefepime if minor ß-lactam
allergy)
Important:
- Review empiric therapy at 48 hours: it may be possible to cease
gentamicin or switch to oral therapy.
- DO NOT USE third generation cephalosporins in HAP.
|
| |
Community-acquired pneumonia (CAP)
Empiric therapy (immunocompetent host)
See JCLIN(VAX) or the HAHS intranet for dosages, advice on investigation
and other alternatives for ß-lactam allergic patients.
Mild/moderate (pneumococcal cover essential)
Oral amoxycillin or doxycycline (not in children aged <8yrs
or pregnant women) or roxithromycin
Parenteral penicillin G
Severe (cover for Legionella and aerobic Gram negatives essential)
Adults, children >10yrs
penicillin G + gentamicin (once daily) + erythromycin (intravenous,
central line)
Children < 10yrs
penicillin G + gentamicin (once daily) + consider flucloxacillin
Important:
- Review empiric therapy at 48 hours: it may be possible to cease
gentamicin or switch to oral therapy.
- A third generation cephalosporin is only indicated in severe CAP
when minor ß-lactam allergy or established renal failure is
present.
These consensus guidelines have been reviewed and accepted
by Paediatric & Adult Respiratory Medicine, ID and Intensive Care
specialists at JHH May 2000. |
At the John Hunter Hospital DUE projects have been conducted twice yearly (in June and December) since 1998, to assess utilisation of third generation cephalosporins and the appropriateness of prescribing. Inappropriate prescribing is defined as that which is outside the hospital anti-infective guidelines and where the infectious diseases physician considers that an alternative anti-infective should have been used. Appropriate prescribing of third generation cephalosporins has increased from 21 per cent to 52 per cent (p=0.008) of courses between December 1999 and June 2001. Whilst this may seem a modest improvement it is in line with the compliance achieved by other workers.3
Surgical prophylaxis
Surgical prophylaxis and wound infection antibiotic guidelines were developed in 2001 after consultation between the AWP and Surgery. These guidelines detail the choice of antibiotic, timing for surgical prophylaxis and the appropriate length of treatment. A laminated card for quick reference was also developed and is promoted and distributed within Surgery. An audit of surgical prophylaxis practices was conducted by the DUE team in March 2000. As a result of this audit agreements have been developed with Gynaecology, relating to cefotetan usage, and with the Cardiac Surgery team regarding vancomycin prophylaxis.
Switch to oral campaign
The increasing trend for antibiotic use overall, and the concern over the complications of intravenous administration, prompted a campaign to encourage the use of oral antibiotics where indicated. 'The Switch to Oral' campaign involved a DUE performed over a 3 week period in November 2001 assessing adult in-patients in non-intensive care wards. Agreed criteria for oral antibiotic use were developed and disseminated to medical staff. Promotional activities included posters and postcards distributed to individual doctors and bright orange stickers placed in the medical charts of patients on intravenous antibiotic orders.
The criteria for eligibility for a switch to oral antibiotics or ceasing of therapy included the following:
- the patient was improving clinically;
- a temperature <38O C for 2 consecutive days;
- oral fluids and food tolerated;
- no ongoing or potential absorption problems;
- no unexplained tachycardia;
- the patient did not have a condition that required high tissue antibiotic concentrations e.g., endocarditis or meningitis; or
- a suitable oral formulation was available.
Of 55 patients fulfilling these criteria, 27 (49%) ceased antibiotics altogether and 18 (33%) switched to a suitable oral form within 3 days. The remaining 10 patients (18%) could have been switched but were not. This is an improvement on a small pilot audit conducted earlier in September 2001 where 74 per cent of 16 eligible patients were switched to an oral alternative. The promotion of the appropriate switch from parenteral to oral antibiotics is ongoing. Outcome measures such as prevalence of antimicrobial resistant organisms within the hospital, monthly antibiotic utilisation data and data relating to complications of parenteral administration (e.g., line sepsis) are being monitored to determine the long-term effects.
Top of page
Nosocomial infection
The prevalence of certain organisms associated with nosocomial infection has dropped since the commencement of intervention to promote appropriate use of antimicrobials at John Hunter Hospital. Between 1997 and 2000, the nosocomial Clostridium difficile infection rate fell from 9.8 per 105 patient days to 4.0 cases per 105 patient days (incidence rate ratio 0.41, 95% CI 0.21-0.80).4 Vancomycin resistant enterococci were first isolated at John Hunter Hospital in 1996. Fourteen isolates occurred in Hunter Area Health hospitals in 1997. Since then, the numbers have decreased, with one urinary vancomycin resistant enterococci isolate in May 2001, and none reported since. Although other factors may be involved, the control of broad-spectrum antibiotic use instituted at John Hunter Hospital may have limited this problem.5
Healthcare-associated acquisition and morbidity due to multiple antibiotic-resistant organisms is closely monitored within the hospital by the Infection Control service. By regular review of the data on antibiotic use and the prevalence of multiple antibiotic-resistant organisms, it was noted that there appeared to be a relationship between the incidence of multi-resistant Acinetobacter baumannii isolation (defined as resistant to gentamicin, ciprofloxacin and carbapenems) and the use of imipenem or meropenem. These antibiotics were being overused in surgery (as empiric treatment for severe pancreatitis), or for severe sepsis in intensive care units. Through an education program, the development of specific guidelines for the use of carbapenems, and strict limitations on the availability of these antibiotics, the use of these agents has decreased. The association between carbapenem (and other antibiotic) usage and multi-resistant Acinetobacter baumannii emergence is being examined further with a formal case-control study.
Top of page
Conclusions
The John Hunter Hospital sought to develop antibiotic guidelines using a multi-disciplinary evidence based approach. With approval from the relevant clinical units, regular evaluations were carried out and individual were feedback was given. The guidelines were disseminated in multiple ways to maximise access by clinical staff. Implementation of the guidelines was via drug bulletins, clinical meetings, educational sessions, and individual contact. Regular review and update of the guidelines was undertaken to ensure relevance. Promotion of appropriate prescribing is an ongoing activity.
This report has described how active promotion of antibiotic guidelines along with educational activities leads to more appropriate prescribing.
Top of page
References
1. Therapeutic Guidelines Limited. Therapeutic Guidelines: Antibiotic. 11th edition. North Melbourne, Australia, 2000.
2. Therapeutic Guidelines Limited. Therapeutic Guidelines: Antibiotic. 10th edition. North Melbourne, Australia, 1998.
3. Robertson MB, Korman TM, Dartnell JG, Ioannides-Demos LL, Kirsa SW, Lord JA, et al. Ceftriaxone and cefotaxime use in Victorian hospitals. Med J Aust 2002:176;524-529.
4. MacDonald J, Ferguson JK. How education influences prescribing at John Hunter Hospital. Australian Prescriber 2001;24:32.
5. Quale J, Landman D, Saurina G, Atwood E, DiTore V, Patel K. Manipulation of a hospital antimicrobial formulary to control an outbreak of vancomycin-resistant enterococci. Clin Infect Dis 1996;23:1020-1025.
Top of page
Author affiliations
1. Department of Microbiology, Hunter Area Pathology Service, John Hunter Hospital, New Lambton, New South Wales
2. Department of Pharmacy, John Hunter Hospital, New Lambton, New South Wales
3. Department of Infectious Disease and Immunology, John Hunter Hospital, New Lambton, New South Wales
Corresponding author: Dr Susan Tiley, Department of Microbiology, Hunter Area Pathology Service, John Hunter Hospital, Lookout Road, New Lambton NSW 2135. Telephone: +61 2 4921 4423. Facsimile: +61 2 4921 4440. Email: stiley@hunter.health.nsw.gov.au
This article was published in Communicable Diseases Intelligence Volume 27 Suppl, May 2003.
