Antibiotics are used both to treat infections in individual patients and in public health interventions to control disease outbreaks. In both circumstances the outcome, as measured by morbidity and mortality, is compromised by antimicrobial resistance (AMR) in the causative organism. Of necessity, antibiotics are frequently given empirically and their selection is based on presumptions of efficacy and the susceptibility of the infecting agent. AMR surveillance provides reassurance with regard to efficacy and guides the formulation of standard treatment regimens. However, AMR surveillance is not always appropriately performed nor are the data generated necessarily used to best advantage. Optimal use of AMR surveillance data requires for each disease of importance: an understanding of the applications of AMR surveillance and a clear definition of the type of data required: the 'triggers for surveillance'; construction of AMR surveillance programs appropriate to differing requirements; and better linkages between AMR surveillance data and disease control functions so that the thresholds for initiating public health action are clearly defined. Examples which illustrate the application of these principles are provided from experience with surveillance of AMR in the pathogenic Neisseria (N. gonorrhoeae and N. meningitidis). Commun Dis Intell 2003;27 Suppl:S70-S74.
Top of page
Introduction
The most obvious application of antibiotic susceptibility testing and surveillance is to facilitate use of the most appropriate treatment in infected individuals. Equally relevant is the role of antibiotics in public health interventions in infectious disease control. Control of certain diseases of public health importance is materially assisted by the ability of antibiotic treatment, either therapeutic or prophylactic, to decrease transmission between individuals and reduce the duration of infectiousness of affected patients. Conversely, increasing or high levels of resistance to the antibiotics used for these purposes pose the very real prospect of increased morbidity and mortality and prolongation of disease outbreaks. This is not to suggest that antibiotic treatment alone is the sole or even major intervention required, but rather that it is one component, albeit a key one, of an integrated public health approach to infectious disease management and control.1
Treatment of an individual infection or use of an antibiotic in a disease outbreak is often commenced before the diagnosis is confirmed and almost always before the susceptibility or resistance to the pathogen can be fully ascertained. In either circumstance, treatments are based on an assumed response to the antibiotic chosen. One important consideration in this choice of agent is the level of resistance to that antibiotic that is likely to be encountered. For example, the use of antibiotics such as rifampicin or ciprofloxacin for prophylaxis in an outbreak of meningococcal disease is predicated on the assumption that the meningococcus remains susceptible to their action. Similarly where a case of invasive meningococcal disease occurs, reassurance that there is no clinically significant resistance in Australia to the penicillins2 means that, in keeping with current recommendations, treatment with this antibiotic can be initiated in general practice before transfer to hospital.
Antimicrobial resistance surveillance thus has an integral place in helping to determine the most appropriate choice of antibiotics for both individual and public health management. It also follows that for public health purposes, this surveillance should be of high quality so that data generated are accurate, and focussed on those diseases and organisms where therapeutic options may be severely limited by antimicrobial resistance (AMR). Further, AMR surveillance for broader public health purposes should be linked with public health disease surveillance and control functions i.e., an effector arm, if value from surveillance is to be fully realised.1
The need for AMR surveillance for public health purposes is therefore based on certain 'triggers' including among others, the importance of the disease in terms of mortality and morbidity, the disease incidence in Australia, and the potential for disease transmission in Australia. The diseases involved would be those where the public health response (in the broad sense) is important and where therapeutic options and disease control are affected by AMR. There are many well established laboratory based programs in Australia for AMR surveillance at a local, national and even international level. What is sometimes lacking however, is a link between these programs and disease surveillance and control. Even when these AMR surveillance systems are in place and links between AMR and disease surveillance are established, there is still a requirement for a definition of 'thresholds for action'. That is, at certain defined and established stages in the evolution of antibiotic resistance, interventions with regard to treatment options, as opposed to separate issues of control of AMR, must be commenced.
To illustrate these principles and their differential applications in various diseases, the example of the place of AMR surveillance in the treatment and control of gonococcal and meningococcal infection in Australia will be used. For Neisseria gonorrhoeae, the specific instance of the emergence and spread of quinolone resistant gonococci in New South Wales is examined. For Neisseria meningitidis the consequences of resistance to antibiotics used for either treatment or prophylaxis would be significant. Examples of surveillance of antibiotic resistance are drawn from published studies, including those of the National Neisseria Network.
Top of page
Methods
Data were derived from the New South Wales component of the Australian Gonococcal Surveillance Programme and the National Neisseria Network. The program of surveillance of antimicrobial resistance in N. gonorrhoeae has been established for over 20 years and is based on the results of examinations of gonococci obtained from public and private sectors.3,4 Quinolone resistant gonococci (QRNG) were subdivided into 'less sensitive' and 'resistant' subgroups on the basis of MIC levels and correlation with patient demographics were as previously described.4,5 The World Health Organization criteria of critical levels of antibiotic resistance in gonococcal populations, namely, a resistance level of 5 per cent or more,6 was used in this example. Meningococcal resistance data for invasive isolates of N. meningitidis in Australia were recently reviewed2 and were also based on data gathered since 1999.7,8 Methods and criteria of resistance have also been previously published.2
Top of page
Results
Surveillance of quinolone antibiotic resistance in N. gonorrhoeae in New South Wales
Quinolone resistance in gonococci was first detected in New South Wales in 1984 but for the next decade generally remained at a low level, was seen almost exclusively in gonococcal infection acquired overseas and was not associated with sustained domestic transmission.9 Quinolone antibiotics, especially ciprofloxacin, were increasingly used successfully in the management of gonorrhoea despite occasional instances of treatment failures.10,11 These cases of treatment failure were infections with gonococci with higher levels of quinolone resistance and again were isolated examples of imported gonococcal disease.
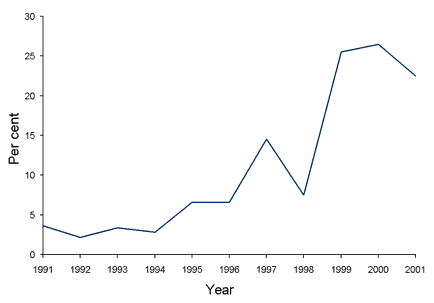
From 1995 to the end of 2001 considerable volatility in the patterns of quinolone resistance was observed in gonococci isolated in New South Wales (Figure) and sustained endemic transmission of QRNG became established. There were several subsets of patients identified with domestic dissemination of QRNG at different times including clients of sex workers and homosexually active males.4,11 The effect of the emergence and local spread of QRNG in New South Wales since 1995 has been a rapid escalation of the rate of QRNG and maintenance of this rate well above the 'critical' 5 per cent level for many years. As a consequence quinolone antibiotics are no longer recommended treatments for gonococcal infection in New South Wales.
Figure. Quinolone resistant gonococci as a percentage of all gonococci isolated in New South Wales, 1991 to 2001
Top of page
Surveillance of antibiotic resistance in N. meningitidis in Australia
The data obtained by the National Neisseria Network from 1994 to 19992 showed a trend towards decreased sensitivity to penicillin in invasive meningococcal isolates in Australia between 1994 and 1996, but no further decrease in sensitivity thereafter.2,7,8 This decrease in susceptibility did not indicate clinical resistance and only two isolates which would be regarded as potentially resistant to the penicillin group of antibiotics were isolated between 1994 and 2001. All isolates examined remained susceptible to the third generation cephalosporin antibiotics. Nine instances of N. meningitidis resistant to rifampicin2,8 and a single quinolone resistant isolate were identified between 1994 and 2001.12
Top of page
Discussion
There are several criteria to be met for establishing meaningful AMR surveillance for public health purposes. The disease must be of public health importance e.g., readily transmissible and of sufficient incidence; therapeutic options and disease control must be affected by AMR and measures must be in place to alter antibiotic treatments when surveillance data reveal a significant change in AMR.
Both of the pathogenic Neisseria, the gonococcus and the meningococcus, warrant active surveillance for emergence and spread of antibiotic resistant strains for public health purposes by the above criteria. Both are of obvious public health importance in terms of incidence, transmissibility, potential morbidity and, in the case of the meningococcus, mortality. Antibiotic therapy is important not only for their treatment but is also integral for disease control. In gonorrhoea, effective antibiotic treatment decreases the duration of infectiousness and the transmissibility of the organism, both key factors in disease control. Public health management of invasive meningococcal disease is heavily reliant on early treatment with an effective antibiotic if the disease is suspected clinically. In Australia, this is with penicillin. Antibiotic prophylaxis is one means of reducing secondary cases of invasive meningococcal disease in close contacts of an index case. However, other agents used for chemoprophylaxis such as sulphonamides have had to be discarded for this application because of antibiotic resistance.2
Australia has well established systems for surveillance of AMR in these two closely related organisms.4,8 Results and analyses of AMR surveillance of both of these organisms are published regularly in Communicable Diseases Intelligence and elsewhere. Despite these similarities, there are important differences in the approaches, principles and methods of AMR surveillance and the public health responses that follow detection of AMR in these two organisms.
With regard to the gonococcus, it is well established that a level of resistance of 5 per cent to an antibiotic in prevalent strains of N. gonorrhoeae should result in that antibiotic being removed from recommended treatment schedules.6 The gonococcus has a particular capacity to become resistant to antibiotics and this has seen the progressive removal of penicillins, tetracyclines and now quinolones from treatment regimens in New South Wales. In other parts of Australia, penicillins continue to be standard treatment because AMR surveillance continues to demonstrate susceptibility to these agents. This '5 per cent' tolerance level is indicative only and in many instances a change in standard treatment would occur at a lower level and in 'high frequency transmitters' of the disease or in small communities, any level of resistance warrants an alteration of recommendations.6 In general, the threshold levels for action as currently defined are such that it is considered sufficient to sample a representative number and distribution of gonococci for public health purposes.
In contrast with gonococci, antibiotic resistance in meningococci in Australia has been slower to develop. Penicillin resistance in meningococci would have wide ranging consequences. Instances of beta-lactamase producing N. meningitidis have been reported overseas and on occasion chemoprophylaxis has been rendered ineffectual.2In vitro models have revealed the potential for meningococci to become resistant to quinolone antibiotics.13 For these reasons and because of the relatively low number of isolates involved, it is necessary to examine all available isolates from invasive cases of meningococcal disease for AMR and to alter treatment schedules sooner rather than later i.e., a 'zero tolerance' approach.
From these examples, it would seem necessary to have in place active surveillance of AMR in the causative organisms of those diseases meeting the criteria outlined above. Just as importantly it is necessary to have the data gathered, critically analysed and interpreted and integrated into wider public health control effector mechanism which includes a plan for action once defined thresholds of AMR are reached. While some of these thresholds of AMR for action have been determined and used for some time e.g., in gonorrhoea, in most instances they remain intuitive and variable e.g., in meningococcal disease. The nature and amount of AMR define these thresholds and predicate the requirements of optimal AMR surveillance for a particular organism, disease and antibiotic combination. For optimal use of AMR surveillance in a public health context, definition and implementation of these thresholds for action is required.
Top of page
Acknowledgements
Athena Limnios, Tiffany Hogan and members of the National Neisseria Network of Australia generated data used in the references below.
Top of page
References
1. World Health Organization. Surveillance standards for antimicrobial resistance. 2001. WHO/CDS/CSR/DRS/2001.5.
2. Tapsall JW, Shultz T, Limnios E, Munro R, Mercer J, Porritt R, et al. Surveillance of antibiotic resistance in invasive isolates of Neisseria meningitidis in Australia, 1994-1999. Pathology 2001;33:359-361.
3. Tapsall JW, Phillips EA, Cossins YM et al. Penicillin sensitivity of gonococci in Australia: the development of an Australian Gonococcal Surveillance Programme. Br J Vener Dis 1984,60:226-230.
4. Australian Gonococcal Surveillance Programme. Annual report of the Australian Gonococcal Surveillance Programme, 2001. Commun Dis Intell 2002;26:242-247.
5. Tapsall JW, Limnios EA, Shultz TR. Continuing evolution of the pattern of quinolone resistance in Neisseria gonorrhoeae isolated in Sydney, Australia. Sex Transm Dis 1998;25:415-417.
6. World Health Organization. Guidelines for the management of sexually transmitted infections. WHO/HIV_AIDS/2001.01 WHO/RHR/01.10:pp 1-5. World Health Organization, Geneva; 2001.
7. Australian Meningococcal Surveillance Programme. Annual report of the Australian Meningococcal Surveillance Programme, 2000. Commun Dis Intell 2001;25:113-121.
8. Australian Meningococcal Surveillance Programme. Annual report of the Australian Meningococcal Surveillance Programme, 2001. Commun Dis Intell 2002;26:407-418.
9. Tapsall JW, Shultz TR, Phillips EA. Characteristics of Neisseria gonorrhoeae isolated in Australia showing decreased sensitivity to quinolone antibiotics. Pathology 1992,24:27-31.
10. Tapsall JW, Shultz TR, Lovett R, Munro R. Failure of 500 mg ciprofloxacin therapy in male urethral gonorrhoea. Med J Aust 1992;156:143.
11. Tapsall JW, Limnios EA, Thacker C, Donovan B, Lynch SD, Kirby LJ, et al. High level quinolone resistance in Neisseria gonorrhoeae: a report of two cases. Sex Transm Dis 1995;22:310-311.
12. Shultz TR, Tapsall JW, White PA, Newton PJ. An invasive isolate of Neisseria meningitidis showing decreased susceptibility to quinolones. Antimicrob Agents Chemother 2000;44:1116.
13. Shultz TR, Tapsall J. GyrA and ParC changes in ciprofloxacin resistant meningococci parallel those seen in gonococci. Abstract 217, Abstract guide of the Twelfth International Pathogenic Neisseria Conference Galveston USA; 12-17 November 2000.
Top of page
Author affiliations
Correspondence: Assoc. Professor John Tapsall, Director, WHO Collaborating Centre for STD and HIV, Microbiology Department, The Prince of Wales Hospital, Randwick NSW 2031. Telephone: +61 2 9382 9079. Facsimile: +61 2 9398 4275. Email: j.tapsall@unsw.edu.au
This article was published in Communicable Diseases Intelligence Volume 27 Suppl, May 2003.
