Top of page
Australian Sentinel Practice Research Network
The Research and Health Promotion Unit of the Royal Australian College of General Practitioners operates the Australian Sentinel Practice Research Network (ASPREN). ASPREN is a national network of general practitioners who report presentations of defined medical conditions each week. The aim of ASPREN is to provide an indicator of the burden of disease in the primary health care setting and to detect trends in consultation rates.
There are currently about 40 general practitioners participating in the network from all states. Seventy-five per cent of these are in metropolitan areas and the remainder are rural based. Between 3,000 and 4,000 consultations are recorded each week.
The list of conditions is reviewed annually by the ASPREN management committee and an annual report is published.
In 2006, six conditions are being monitored, four of which are related to communicable diseases. These include influenza, gastroenteritis, varicella and shingles. Definitions of these conditions are described in Surveillance systems reported in CDI, published in Commun Dis Intell 2006;30:158. Note that in 2006, two case definitions for influenza are being recorded in parallel.
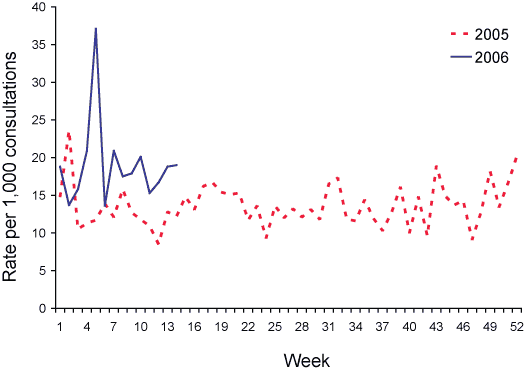
Data from 1 January to 31 March 2006 are shown as the rate per 1,000 consultations in Figures 4 and 5.
Figure 4. Consultation rates for influenza-like illness, ASPREN, 1 January to 31 March 2006, by week of report
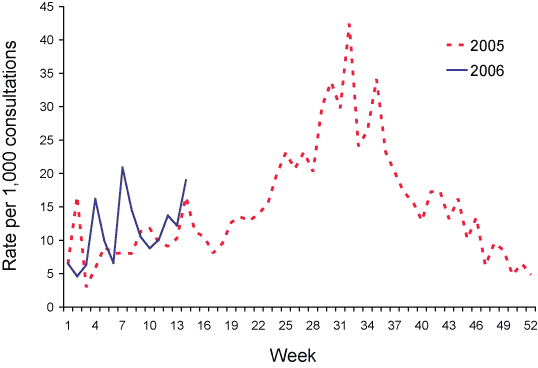
Figure 5. Consultation rates for gastroenteritis, ASPREN, 1 January to 31 March 2006, by week of report
Top of page
Meningococcal surveillance
John Tapsall, The Prince of Wales Hospital, Randwick, NSW, 2031 for the Australian Meningococcal Surveillance Programme.
The reference laboratories of the Australian Meningococcal Surveillance Programme report data on the number of laboratory confirmed cases confirmed either by culture or by non-culture based techniques. Culture positive cases, where a Neisseria meningitidis is grown from a normally sterile site or skin, and non-culture based diagnoses, derived from results of nucleic acid amplification assays and serological techniques, are defined as invasive meningococcal disease (IMD) according to Public Health Laboratory Network definitions. Data contained in the quarterly reports are restricted to a description of the number of cases per jurisdiction, and serogroup, where known. A full analysis of laboratory confirmed cases of IMD is contained in the annual reports of the Programme, published in Communicable Diseases Intelligence. For more information about the program see Surveillance systems reported in CDI, published in Commun Dis Intell 2006;30:157.
Laboratory-confirmed cases of invasive meningococcal disease for the period 1 January to 31 March 2006, are included in this issue of Communicable Diseases Intelligence (Table 6).
Table 6. Number of laboratory confirmed cases of invasive meningococcal disease, Australia, 1 January to 31 March 2006, by jurisdiction and serogroup
Jurisdiction |
Year |
Serogroup |
| A |
B |
C |
Y |
W135 |
ND |
All |
| Q1 |
ytd |
Q1 |
ytd |
Q1 |
ytd |
Q1 |
ytd |
Q1 |
ytd |
Q1 |
ytd |
Q1 |
ytd |
| Australian Capital Territory |
06 |
|
|
|
|
|
|
|
|
|
|
|
|
0 |
0 |
05 |
|
|
1 |
1 |
1 |
1 |
|
|
|
|
|
|
2 |
2 |
04 |
|
|
|
|
2 |
2 |
|
|
|
|
|
|
2 |
2 |
| Northern Territory |
06 |
|
|
1 |
1 |
|
|
|
|
|
|
|
|
1 |
1 |
05 |
|
|
1 |
1 |
|
|
|
|
|
|
|
|
1 |
1 |
04 |
|
|
3 |
3 |
|
|
|
|
|
|
|
|
3 |
3 |
| New South Wales |
06 |
|
|
9 |
9 |
1 |
1 |
|
|
1 |
1 |
4 |
4 |
14 |
14 |
05 |
|
|
15 |
15 |
7 |
7 |
1 |
1 |
|
|
1 |
1 |
|
24 |
04 |
|
|
19 |
19 |
5 |
5 |
1 |
1 |
1 |
1 |
5 |
5 |
31 |
31 |
| Queensland |
06 |
|
|
15 |
15 |
1 |
1 |
|
|
|
|
|
|
16 |
16 |
05 |
|
|
10 |
10 |
5 |
5 |
|
|
|
|
|
|
15 |
15 |
04 |
|
|
12 |
12 |
7 |
7 |
|
|
|
|
2 |
2 |
21 |
21 |
| South Australia |
06 |
|
|
3 |
3 |
|
|
|
|
|
|
|
|
3 |
3 |
05 |
|
|
|
|
2 |
2 |
|
|
|
|
|
|
2 |
2 |
04 |
|
|
4 |
4 |
|
|
|
|
|
|
|
|
4 |
4 |
| Tasmania |
06 |
|
|
1 |
1 |
1 |
1 |
|
|
|
|
|
|
2 |
2 |
05 |
|
|
|
|
|
|
|
|
|
|
|
|
0 |
0 |
04 |
|
|
2 |
2 |
|
|
|
|
|
|
2 |
2 |
4 |
4 |
| Victoria |
06 |
|
|
10 |
10 |
2 |
2 |
1 |
1 |
2 |
2 |
|
|
15 |
15 |
05 |
|
|
7 |
7 |
1 |
1 |
|
|
2 |
2 |
1 |
1 |
11 |
11 |
04 |
|
|
9 |
9 |
4 |
4 |
2 |
2 |
|
|
1 |
1 |
16 |
16 |
| Western Australia |
06 |
|
|
5 |
5 |
|
|
|
|
|
|
|
|
5 |
5 |
05 |
|
|
5 |
5 |
|
|
1 |
1 |
|
|
|
|
6 |
6 |
04 |
|
|
4 |
4 |
1 |
1 |
|
|
|
|
|
|
5 |
5 |
| Australia |
06 |
|
|
44 |
44 |
5 |
5 |
1 |
1 |
3 |
3 |
4 |
4 |
57 |
57 |
05 |
|
|
39 |
39 |
16 |
16 |
2 |
2 |
2 |
2 |
2 |
2 |
61 |
61 |
04 |
|
|
53 |
53 |
19 |
19 |
3 |
3 |
1 |
1 |
10 |
10 |
86 |
86 |
Top of page
HIV and AIDS surveillance
National surveillance for HIV disease is coordinated by the National Centre in HIV Epidemiology and Clinical Research (NCHECR), in collaboration with State and Territory health authorities and the Commonwealth of Australia. Cases of HIV infection are notified to the National HIV Database on the first occasion of diagnosis in Australia, by either the diagnosing laboratory (Australian Capital Territory, New South Wales, Tasmania, Victoria) or by a combination of laboratory and doctor sources (Northern Territory, Queensland, South Australia, Western Australia). Cases of AIDS are notified through the State and Territory health authorities to the National AIDS Registry. Diagnoses of both HIV infection and AIDS are notified with the person's date of birth and name code, to minimise duplicate notifications while maintaining confidentiality.
Tabulations of diagnoses of HIV infection and AIDS are based on data available three months after the end of the reporting interval indicated, to allow for reporting delay and to incorporate newly available information. More detailed information on diagnoses of HIV infection and AIDS is published in the quarterly Australian HIV Surveillance Report, and annually in 'HIV/AIDS, viral hepatitis and sexually transmissible infections in Australia, annual surveillance report'. The reports are available from the National Centre in HIV Epidemiology and Clinical Research, 376 Victoria Street, Darlinghurst NSW 2010. Internet: http://www.med.unsw.edu.au/nchecr. Telephone: +61 2 9332 4648. Facsimile: +61 2 9332 1837. For more information see Surveillance systems reported in CDI, published in Commun Dis Intell 2006;30:159.
HIV and AIDS diagnoses and deaths following AIDS reported for 1 October to 31 December 2005, as reported to 31 March 2006, are included in this issue of Communicable Diseases Intelligence (Tables 7 and 8).
Table 7. New diagnoses of HIV infection, new diagnoses of AIDS and deaths following AIDS occurring in the period 1 October to 31 December 2005, by sex and state or territory of diagnosis
| |
Sex |
State or territory |
This period 2005 |
This period 2004 |
YTD 2005 |
YTD 2004 |
| ACT |
NSW |
NT |
Qld |
SA |
Tas |
Vic |
WA |
| HIV diagnoses |
Female |
0 |
4 |
0 |
2 |
2 |
0 |
9 |
3 |
20 |
35 |
95 |
121 |
| Male |
0 |
88 |
0 |
33 |
11 |
0 |
64 |
13 |
209 |
193 |
861 |
774 |
| Not reported |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
| Total* |
0 |
92 |
0 |
35 |
13 |
0 |
73 |
16 |
229 |
228 |
956 |
897 |
| AIDS diagnoses |
Female |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
1 |
7 |
26 |
21 |
| Male |
0 |
12 |
0 |
9 |
1 |
0 |
12 |
3 |
37 |
42 |
168 |
156 |
| Total* |
0 |
12 |
1 |
9 |
1 |
0 |
12 |
3 |
38 |
49 |
194 |
179 |
| AIDS deaths |
Female |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
1 |
5 |
7 |
| Male |
0 |
5 |
0 |
4 |
1 |
0 |
3 |
1 |
14 |
27 |
56 |
83 |
| Total* |
0 |
7 |
0 |
4 |
1 |
0 |
3 |
1 |
16 |
28 |
61 |
90 |
Table 8. Cumulative diagnoses of HIV infection, AIDS and deaths following AIDS since the introduction of HIV antibody testing to 1 October to 31 December 2005, and reported by 31 March 2006,by sex and state or territory
| |
Sex |
State or territory |
Total |
| ACT |
NSW |
NT |
Qld |
SA |
Tas |
Vic |
WA |
| HIV diagnoses |
Female |
30 |
819 |
18 |
244 |
89 |
8 |
341 |
182 |
1,731 |
| Male |
252 |
13,096 |
125 |
2,592 |
881 |
95 |
4,993 |
1,157 |
23,191 |
| Not reported |
0 |
231 |
0 |
0 |
0 |
0 |
22 |
0 |
253 |
| Total* |
282 |
14,174 |
143 |
2,845 |
971 |
103 |
5,375 |
1,346 |
25,239 |
| AIDS diagnoses |
Female |
10 |
244 |
3 |
68 |
31 |
4 |
105 |
36 |
501 |
| Male |
92 |
5,296 |
41 |
1,010 |
393 |
50 |
1,925 |
418 |
9,225 |
| Total* |
102 |
5,557 |
44 |
1,080 |
425 |
54 |
2,040 |
456 |
9,758 |
| AIDS deaths |
Female |
7 |
134 |
1 |
41 |
20 |
2 |
59 |
24 |
288 |
| Male |
71 |
3,552 |
26 |
652 |
273 |
32 |
1,385 |
292 |
6,283 |
| Total |
78 |
3,696 |
27 |
695 |
293 |
34 |
1,452 |
317 |
6,592 |
Top of page
Childhood immunisation coverage
Tables 9, 10 and 11 provide the latest quarterly report on childhood immunisation coverage from the Australian Childhood Immunisation Register (ACIR).
The data show the percentage of children fully immunised at age 12 months for the cohort born between 1 October and 31 December 2004; at 24 months of age for the cohort born between 1 October and 31 December 2003; and at 6 years of age for the cohort born between 1 October and 31 December 1999, according to the Australian Standard Vaccination Schedule.
For information about the Australian Childhood Immunisation Register see Surveillance systems reported in CDI, published in Commun Dis Intell 2006;30:157 and for a full description of the methodology used by the Register see Commun Dis Intell 1998;22:36-37.
Commentary on the trends in ACIR data is provided by the National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases (NCIRS). Telephone: +61 2 9845 1256. Email: brynleyh@chw.edu.au.
Immunisation coverage for children ‘fully immunised’ at 12 months of age for Australia decreased for the first time in 12 months, a drop of 0.8 percentage points to 90.2 per cent (Table 9). Coverage for all individual vaccines due at 12 months of age decreased by 0.5–0.7 percentage points. The only significant movements in coverage for individual vaccines by jurisdiction was in Tasmania, where coverage for all four vaccines due at 12 months decreased by 1.6–2.2 percentage points.
Table 9. Percentage of children immunised at 1 year of age, preliminary results by vaccine and state or territory for the birth cohort 1 October and 31 December 2004; assessment date 31 March 2006
| |
State or territory |
|
| |
ACT |
NSW |
NT |
Qld |
SA |
Tas |
Vic |
WA |
Australia |
| Number of children |
1,019 |
21,277 |
774 |
12,317 |
4,263 |
1,408 |
15,517 |
6,012 |
62,587 |
| Diphtheria, tetanus, pertussis (%) |
92.9 |
91.7 |
91.9 |
91.4 |
91.8 |
92.8 |
92.1 |
90.8 |
91.7 |
| Poliomyelitis (%) |
92.8 |
91.6 |
91.7 |
91.4 |
91.7 |
92.8 |
92.0 |
90.7 |
91.6 |
| Haemophilus influenzae type b (%) |
94.9 |
93.5 |
96.4 |
93.6 |
94.4 |
93.4 |
94.1 |
93.7 |
93.8 |
| Hepatitis B (%) |
95.6 |
94.6 |
96.8 |
94.1 |
94.7 |
93.5 |
94.0 |
93.7 |
94.3 |
| Fully immunised (%) |
92.2 |
90.0 |
91.5 |
90.3 |
90.6 |
91.2 |
90.3 |
89.3 |
90.2 |
| Change in fully immunised since last quarter (%) |
-1.6 |
-0.6 |
+1.4 |
-0.8 |
-0.7 |
-2.2 |
-1.7 |
+0.6 |
-0.8 |
Immunisation coverage for children ‘fully immunised’ at 24 months of age for Australia did not change from the last quarter, remaining at 92.1 per cent (Table 10). Similarly, there were no significant changes in coverage in any jurisdiction for ‘fully immunised’ coverage or for coverage for individual vaccines.
Table 10. Percentage of children immunised at 2 years of age, preliminary results by vaccine and state or territory for the birth cohort 1 October and 31 December 2003, assessment date 31 March 2006
| |
State or territory |
|
| |
ACT |
NSW |
NT |
Qld |
SA |
Tas |
Vic |
WA |
Australia |
| Number of children |
1,086 |
21,739 |
849 |
12,867 |
4,424 |
1,501 |
15,926 |
6,269 |
64,661 |
| Diphtheria, tetanus, pertussis (%) |
95.8 |
95.0 |
96.8 |
94.7 |
95.1 |
97.0 |
95.8 |
93.6 |
95.1 |
| Poliomyelitis (%) |
95.5 |
94.9 |
96.7 |
94.7 |
95.1 |
97.1 |
95.7 |
93.6 |
95.0 |
| Haemophilus influenzae type b (%) |
93.7 |
93.1 |
95.1 |
93.5 |
93.9 |
95.1 |
94.4 |
91.6 |
93.5 |
| Measles, mumps, rubella (%) |
93.5 |
93.4 |
95.9 |
93.3 |
94.3 |
95.5 |
94.9 |
92.4 |
93.8 |
| Hepatitis B(%) |
96.2 |
95.9 |
97.5 |
95.3 |
96.1 |
97.8 |
96.5 |
94.9 |
95.9 |
| Fully immunised (%) |
92.1 |
91.6 |
94.4 |
91.8 |
92.7 |
94.4 |
93.2 |
90.1 |
92.1 |
| Change in fully immunised since last quarter (%) |
-2.7 |
-0.1 |
+1.2 |
-0.1 |
+1.7 |
-0.0 |
+0.8 |
-1.3 |
-0.0 |
Top of page
Table 11 shows immunisation coverage estimates for ‘fully immunised’ and for individual vaccines at 6 years of age for Australia by state or territory. This was largely unchanged in all jurisdictions except for Tasmania. Coverage for all vaccines due at 6 years of age in Tasmania decreased by 3 percentage points. However, Tasmania is not a large jurisdiction in terms of population and has experienced such changes in coverage, in both directions, on numerous occasions since coverage at 6 years of age was first reported in 2002.
Table 11. Percentage of children immunised at 6 years of age, preliminary results by vaccine and state or territory for the birth cohort 1 October and 31 December 1999; assessment date 31 March 2006
| |
State or territory |
|
| |
ACT |
NSW |
NT |
Qld |
SA |
Tas |
Vic |
WA |
Australia |
| Number of children |
984 |
21,547 |
789 |
12,942 |
4,563 |
1,573 |
15,913 |
6,612 |
64,923 |
| Diphtheria, tetanus, pertussis (%) |
87.8 |
85.2 |
82.8 |
83.1 |
83.5 |
84.5 |
88.0 |
80.7 |
84.9 |
| Poliomyelitis (%) |
88.8 |
85.0 |
83.4 |
83.2 |
83.6 |
84.6 |
87.9 |
80.3 |
84.8 |
| Measles, mumps, rubella (%) |
88.2 |
85.1 |
83.9 |
83.4 |
83.7 |
84.6 |
88.0 |
80.4 |
84.9 |
| Fully immunised (%) |
87.0 |
84.1 |
82.0 |
81.8 |
82.6 |
83.6 |
87.1 |
79.1 |
83.8 |
| Change in fully immunised since last quarter (%) |
-1.2 |
-0.6 |
-1.1 |
+0.4 |
+0.8 |
-3.0 |
-0.2 |
-0.5 |
-0.2 |
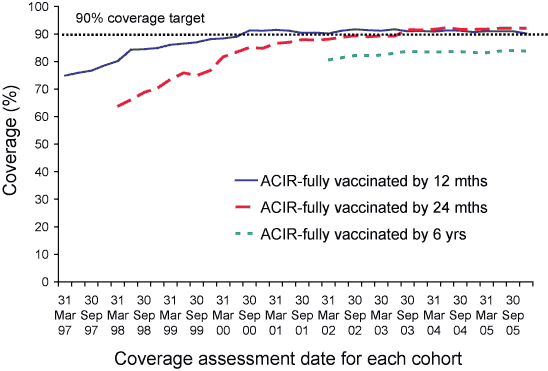
Figure 6 shows the trends in vaccination coverage from the first ACIR-derived published coverage estimates in 1997 to the current estimates. There is a clear trend of increasing vaccination coverage over time for children aged 12 months, 24 months and 6 years, although the rate of increase has slowed over the past two years for all age groups. The Figure shows that there have now been 10 consecutive quarters where ‘fully immunised’ coverage at 24 months of age has been greater than ‘fully immunised’ coverage at 12 months of age, following the removal of the requirement for the 18-month DTPa vaccine. However, both measures have been above 90 per cent for this 27-month period and show levels of high coverage for the vaccines included have been maintained over a significant period of time. Currently, coverage for the more recent vaccines, meningococcal C conjugate at 12 months and pneumococcal conjugate at 2, 4, and 6 months, are not included in the 12 or 24 months coverage data.
Figure 6. Trends in vaccination coverage, Australia, 1997 to 2005, by age cohorts
Acknowledgement: These figures were provided by Medicare Australia, to specifications provided by the Australian Government Department of Health and Ageing. For further information on these figures or data on the Australian Childhood Immunisation Register please contact the Immunisation Section of Medicare Australia: Telephone: +61 2 6124 6607.
Top of page
National Enteric Pathogens Surveillance System
The National Enteric Pathogens Surveillance System (NEPSS) collects, analyses and disseminates data on human enteric bacterial infections diagnosed in Australia. Communicable Diseases Intelligence NEPSS quarterly reports include only Salmonella. NEPSS receives reports of Salmonella isolates that have been serotyped and phage typed by the six Salmonella laboratories in Australia. Salmonella isolates are submitted to these laboratories for typing by primary diagnostic laboratories throughout Australia.
A case is defined as the isolation of a Salmonella from an Australian resident, either acquired locally or as a result of overseas travel, including isolates detected during immigrant and refugee screening. Second and subsequent identical isolates from an individual within six months are excluded, as are isolates from overseas visitors to Australia. The date of the case is the date the primary diagnostic laboratory isolated Salmonella from the clinical sample.
Quarterly reports include historical quarterly mean counts. These should be interpreted cautiously as they may be affected by outbreaks and by surveillance artefacts such as newly recognised and incompletely typed Salmonella.
NEPSS may be contacted at the Microbiological Diagnostic Unit, Public Health Laboratory, Department of Microbiology and Immunology, The University of Melbourne; by telephone: +61 3 8344 5701, facsimile: +61 3 8344 7833 or email joanp@unimelb.edu.au
Scientists, diagnostic and reference laboratories contribute data to NEPSS, which is supported by state and territory health departments and the Australian Government Department of Health and Ageing.
Reports to the National Enteric Pathogens Surveillance System of Salmonella infection for the period 1January to 31 March 2006 are included in Tables 11and 12. Data include cases reported and entered by 24 April 2006. Counts are preliminary, and subject to adjustment after completion of typing and reporting of further cases to NEPSS. For more information see Commun Dis Intell 2006;30:159–160.
First quarter 2006
The total number of reports to NEPSS of human Salmonella infection rose to 2,876 in the first quarter of 2006, 31 per cent more than in fourth quarter of 2005. The first quarter count was seven per cent more than the comparable first quarter of 2005 and approximately 12 per cent greater than the ten-year historical mean for this period. Indeed, the 2,986 reports represent the second highest count for any quarter since at least 1991.
A wide range of salmonellae have contributed to this excess of cases, including those associated with outbreaks and unseasonable increases in S. Typhimurium phage type 135 (widespread), S. Typhimurium phage type 44 (Victoria and New South Wales), S. Oranienberg (Western Australia), and S. Bovismorbificans phage type 24 (eastern states). More modest recent increases have involved S. Birkenhead (northern New South Wales), S. Infantis (New South Wales and South Australia), S. Hvittingfoss (Victoria and Queensland), S. Anatum (South Australia), S. Potsdam (New South Wales), and S. Virchow phage type 25 var 1 (Queensland). The sustained elevation in disease due to the related S. Typhimurium phage types 170 and 108 continues.
During the first quarter of 2006, the 25 most common Salmonella types in Australia accounted for 1,888 cases, 66 per cent of all reported human Salmonella infections. Twenty-two of the 25 most common Salmonella infections in the first quarter of 2006 were also among the 25 most commonly reported in preceding quarter.
Table 11. Reports to the National Enteric Pathogens Surveillance System of Salmonella isolated from humans during the period 1 January to 31 March 2006, as reported to 24 April 2006
| |
State or territory |
| ACT |
NSW |
NT |
Qld |
SA |
Tas |
Vic |
WA |
Australia |
| Total all Salmonella for quarter |
37 |
741 |
91 |
993 |
180 |
81 |
473 |
280 |
2,876 |
| Total contributing Salmonella types |
22 |
146 |
41 |
134 |
53 |
10 |
103 |
72 |
253 |
Table 12. Top 25 Salmonella types identified in Australia, 1 January to 31 March 2006, by state or territory
| National rank |
Organism name |
State or territory |
Total 1st quarter 2006 |
Last 10 years mean 1st quarter |
Year to date 2006 |
Year to date 2005 |
| ACT |
NSW |
NT |
Qld |
SA |
Tas |
Vic |
WA |
1 |
S. Typhimurium PT 135 |
5 |
79 |
0 |
52 |
10 |
14 |
72 |
24 |
255 |
233 |
255 |
129 |
2 |
S. Typhimurium PT 170 |
3 |
97 |
0 |
24 |
0 |
11 |
30 |
0 |
165 |
81 |
165 |
165 |
3 |
S. Saintpaul |
1 |
14 |
8 |
93 |
2 |
0 |
15 |
23 |
156 |
129 |
156 |
157 |
4 |
S. Typhimurium PT 9 |
4 |
25 |
0 |
23 |
19 |
8 |
69 |
6 |
154 |
180 |
154 |
166 |
5 |
S. Birkenhead |
1 |
46 |
0 |
63 |
0 |
0 |
3 |
0 |
113 |
96 |
113 |
71 |
6 |
S. Virchow PT 8 |
1 |
9 |
2 |
94 |
0 |
0 |
2 |
1 |
109 |
93 |
109 |
102 |
7 |
S. Oranienburg |
1 |
5 |
0 |
3 |
2 |
0 |
2 |
69 |
82 |
17 |
82 |
13 |
8 |
S. Typhimurium PT 44 |
0 |
18 |
0 |
12 |
4 |
4 |
33 |
4 |
75 |
19 |
75 |
5 |
9 |
S. Infantis |
2 |
29 |
3 |
8 |
13 |
0 |
7 |
2 |
64 |
46 |
64 |
52 |
10 |
S. Aberdeen |
0 |
2 |
0 |
59 |
0 |
0 |
2 |
1 |
64 |
43 |
64 |
65 |
11 |
S. Hvittingfoss |
1 |
3 |
2 |
40 |
0 |
0 |
17 |
0 |
63 |
35 |
63 |
55 |
12 |
S. Chester |
0 |
11 |
1 |
36 |
2 |
0 |
3 |
9 |
62 |
67 |
62 |
74 |
13 |
S. Mississippi |
2 |
3 |
0 |
5 |
1 |
39 |
3 |
2 |
55 |
38 |
55 |
31 |
14 |
S. Waycross |
1 |
14 |
0 |
39 |
0 |
0 |
0 |
0 |
54 |
46 |
54 |
44 |
15 |
S. Muenchen |
0 |
11 |
4 |
23 |
1 |
0 |
2 |
9 |
50 |
59 |
50 |
65 |
16 |
S. Anatum |
0 |
4 |
3 |
13 |
18 |
0 |
7 |
5 |
50 |
32 |
50 |
19 |
17 |
S. Bovismorbificans PT 24 |
0 |
15 |
1 |
23 |
3 |
0 |
8 |
0 |
50 |
3.9 |
50 |
6 |
18 |
S. Typhimurium RDNC |
1 |
12 |
1 |
5 |
6 |
1 |
12 |
4 |
42 |
30 |
42 |
31 |
19 |
S. Potsdam |
2 |
15 |
0 |
17 |
3 |
0 |
3 |
1 |
41 |
20 |
41 |
9 |
20 |
S. Typhimurium PT 197 |
0 |
9 |
0 |
24 |
1 |
0 |
5 |
0 |
39 |
56 |
39 |
383 |
21 |
S. Typhimurium PT 12 |
0 |
17 |
0 |
4 |
4 |
0 |
4 |
8 |
37 |
32 |
37 |
56 |
22 |
S. Typhimurium untypable |
0 |
4 |
0 |
2 |
0 |
1 |
13 |
8 |
28 |
20 |
28 |
15 |
23 |
S. Weltevreden |
0 |
4 |
4 |
13 |
2 |
0 |
3 |
1 |
27 |
11 |
27 |
14 |
24 |
S. Virchow PT 25 var 1 |
0 |
1 |
0 |
25 |
0 |
0 |
1 |
0 |
27 |
0.6 |
27 |
6 |
25 |
S. Typhimurium PT 108 |
0 |
3 |
0 |
1 |
22 |
0 |
0 |
0 |
26 |
11 |
26 |
20 |
Acknowledgement: We thank scientists, contributing laboratories, state and territory health departments, and the Australian Government Department of Health and Ageing for their contributions to NEPSS.
This report was published in Communicable Diseases Intelligence Vol 30 No 2, June 2006.
