Surveillance of influenza in Australia is based on laboratory isolation of influenza viruses, sentinel general-practitioner reports of influenza-like illness, and absenteeism data from a major national employer. In 2005, 4,575 cases of laboratory-confirmed influenza-like illness were reported, which was 115 per cent higher than in 2004. The influenza season started in the first week of June, with peak activity in early August, a month earlier than in 2004. Influenza A was the predominant type notified (73%), while influenza B activity continued to increase compared to previous years. During 2005, the influenza notification rate amongst persons aged over 65 years (22 cases per 100,000 population) was 70 per cent higher than the mean rate of the last four years. One thousand one hundred and seventy-four influenza isolates from Australia were antigenically analysed: 689 were A(H3N2), 210 were A(H1N1) strains and 275 were influenza B viruses. Continued antigenic drift was seen with the A(H3N2) viruses from the previous reference strains with approximately one quarter of isolates being distinguishable from A/Wellington/1/2004-like viruses and more closely matched to A/California/7/2004-like viruses. Commun Dis Intell 2006;30:189–200.
Top of page
Introduction
Influenza is a major threat to public health worldwide because of its ability to spread rapidly through populations, showing a greater severity in the very young, the frail elderly and people with chronic diseases.
Influenza is an acute self-limiting viral disease of the upper respiratory tract. The health and economic impact of influenza largely arise from related complications such as lower respiratory tract infections and exacerbation of cardiopulmonary and other chronic diseases. These complications result in excess hospitalisation and mortality.
Influenza infections are seasonal in temperate climates (June to September in the Southern Hemisphere and December to April in the Northern Hemisphere), but may occur throughout the year in tropical regions. The seasonal activity of influenza virus varies from year to year with some years marked by larger epidemics with higher morbidity and mortality. In Australia in 2004, influenza and pneumonia were the underlying causes of 3,381 deaths.1
The potential for an epidemic of influenza is dependent on the susceptibility of the population and the ability of the viruses to evolve. There are three types of influenza viruses, A, B and C. The ancestral hosts for influenza A viruses are aquatic birds, however, certain subtypes have become established in various mammals, including humans and pigs. Both influenza B and C are restricted to humans, although influenza C has been isolated from pigs.2 Influenza C causes a less severe illness than either influenza A or B, more akin to the common cold.3
Influenza virus types are further subtyped by the antigenic properties of two surface proteins, haemagglutinin (H) and neuraminidase (N). Influenza viruses are successful human pathogens because of their ability to vary these two external proteins, thus evading the immune system. Mutations cause a gradual change in these proteins called 'antigenic drift', which results in annual epidemics of influenza. The greater the change in these proteins, the more likely it is that the virus will evade the immunity conferred by earlier infections or vaccinations, and the greater the epidemic potential.
At irregular intervals, there are more dramatic changes in the viral proteins, called 'antigenic shift', which are a result of either direct introduction of avian influenza viruses into the human population or a reassortment between human and avian viruses which is postulated to occur in an intermediate host such as pigs. Periodically, these 'shifts' result in the emergence of a novel influenza virus that spreads rapidly through susceptible populations, resulting in pandemics (worldwide epidemics).4 Unlike the seasonal epidemics of influenza, where attack rates depend on age, reflecting immunity conferred from previous infection, in pandemic influenza all age groups are susceptible.
Three pandemics occurred in the 20th century. The Spanish Flu (A/H1N1) pandemic of 1918–1919, is estimated to have caused at least 20 million deaths worldwide, with unusually high mortality among young adults.5 Mortality associated with the 1957 'Asian Flu' (A/H2N2) and the 1968 'Hong Kong Flu' (A/H3N2) pandemics was less severe, with the highest mortality in the elderly and persons with chronic diseases.6
As it is impossible to predict when the next pandemic will occur or how severe the illness will be, an effective national surveillance system is essential for the control of seasonal epidemics and preparedness for potential pandemics. Since 2003, outbreaks of influenza A(H5N1) virus in multiple bird species in Asia, Europe and Africa and associated human and animal cases have raised serious concerns that this virus subtype may acquire the ability for person-to-person transmission and result in pandemic influenza (http://www.who.int/csr/disease/avian_influenza/en/index.html). Virological and epidemiological monitoring are important components of influenza surveillance. The main objectives of influenza surveillance are:
- early detection of epidemics to enable the implementation of public health measures such as the vaccination of high risk groups, outbreak control campaigns and provisions of clinical services;
- characterisation of the nature of the epidemic;
- isolation and antigenic characterisation of circulating influenza viruses to assist in the formulation of the following season's vaccine and to provide new vaccine strains; and
- evaluation of the impact of the epidemic and associated public health measures.
In 2005, the Communicable Diseases Australia website (http://www.health.gov.au/cda) published influenza surveillance data fortnightly during the influenza season. This annual influenza report is a summary of the Australian surveillance information gathered by various systems in 2005, and includes a summary of related international influenza activity.
Top of page
Surveillance methods
Surveillance of influenza in Australia is based on six sets of data:
- notifications of laboratory-confirmed influenza required by legislation in most states and territories, and notified to the National Notifiable Diseases Surveillance System (NNDSS);
- laboratory diagnosis including virus isolation and serology by laboratories participating in the Laboratory Virology and Serology Reporting Scheme (LabVISE);
- subtype and strain data of circulating influenza viruses provided by the World Health Organization (WHO) Collaborating Centre for Reference and Research on Influenza;
- consultation rates for influenza-like illness diagnosed by sentinel general practitioners;
- absenteeism data of workers from a national employer; and
- hospitalisation and mortality data.
National Notifiable Diseases Surveillance System
In all jurisdictions except South Australia, laboratory-confirmed influenza is a notifiable disease under state and territory legislature. Although influenza is not a notifiable condition in South Australia, laboratory reports are collected and sent to NNDSS. In this report, data are analysed by the date of onset in order to present disease occurrence during the reporting period, but when this was not available the earliest date from specimen collection date and notification date was used.
Laboratory surveillance
LabVISE is a national scheme of sentinel laboratories that report influenza diagnosis all year round. In 2005, 11 laboratories from all jurisdictions except Western Australia and the Northern Territory contributed to the scheme. Data were reported to LabVISE monthly and analysed by specimen collection date.
Sentinel general practitioner surveillance
Sentinel general practitioner surveillance schemes for influenza monitor the consultation rates for influenza-like-illness (ILI). In Australia, there are six such schemes: the Australian Sentinel Practice Research Network (ASPREN) which collects data at a national level, the New South Wales Influenza Surveillance Program, the Queensland Influenza-like Illness Sentinel Surveillance in General Practice Program, the Victorian Influenza Surveillance Scheme, Western Australian sentinel general practices, and the Northern Territory Tropical Influenza Surveillance Scheme. ASPREN and the Northern Territory Tropical Influenza Surveillance Scheme report ILI rates throughout the year, while the other sentinel surveillance schemes report from May to October each year.
The national case definition of ILI is: presentation with fever, cough and fatigue. All sentinel surveillance schemes, including ASPREN, used the national case definition for ILI in 2005.
Absenteeism surveillance
Australia Post, a major nation-wide employer, provided sick leave absenteeism data collected weekly for 2005. Absenteeism, defined as an absence due to illness for at least three consecutive days, was presented as a rate per 100 employees per week, on an average of 32,938 employees per week.
Top of page
WHO Collaborating Centre for Reference and Research on Influenza
The WHO Collaborating Centres for Reference and Research on Influenza located in Australia, Japan, the United Kingdom and the United States of America, are responsible for analysing influenza viruses collected through an international surveillance network involving over 100 national laboratories. The Melbourne centre analyses viruses received from Australia and from laboratories throughout Oceania, the Asian region and beyond. All virus isolates are analysed antigenically and a geographically and temporally representative sample, together with any strains demonstrating uncharacteristic reactions during antigenic characterisation, are further analysed by genetic sequencing of the viral haemagglutinin antigen and, for a proportion of these, the neuraminidase antigen. Together with serological and epidemiological data, this forms the basis from which WHO makes recommendations in February (Northern Hemisphere) and September (Southern Hemisphere) for the formulation of vaccines to be used in the following winter.
WHO vaccine formulation recommendations are made in the context of strains that are antigenically 'like' laboratory reference strains that are named according to a standard nomenclature for influenza viruses. For human isolates this nomenclature is based on type, the place of isolation, sequential number and year of isolation; for influenza A the subtype of the haemagglutinin (H) and neuraminidase (N) proteins may be included in brackets after the designation. For avian/animal isolates the species yielding the isolate is also included. An example of a human isolate is A/Sydney/5/97(H3N2), an influenza A(H3N2) virus that was the 5th sequential influenza A isolated in Sydney for the year in 1997. The WHO recommendations (e.g. recommendations for 2006; http://www.who.int/csr/disease/influenza/vaccinerecommendations1/en/index.html) are then translated into actual virus strains acceptable to regulatory authorities and vaccine manufacturers by national and regional committees (e.g. the Australian Influenza Vaccine Committee http://www.tga.gov.au/about/committees-aivc.htm).
Hospitalisation data
The Australian Institute of Health and Welfare provided data on hospital separations in public and private hospitals. The number of separations with a primary diagnosis of influenza due to identified influenza viruses (ICD-10AM = J10) and influenza where the virus was not identified (ICD-10AM = J11) were reported for 2004. Data were not available for 2005 at the time of writing this report.
Adult Vaccination Survey
In past years, vaccination coverage rates for over 65 year olds were reported from the Adult Vaccination Survey (AVS). In 2005, the AVS was not conducted due to the anticipated effect of influenza vaccine supply problems on the results. Therefore coverage of the Influenza Vaccine Program for Older Australians was difficult to estimate. During 2005, numerous eligible over 65 year olds chose to buy their influenza vaccine through the Pharmaceutical Benefits Scheme (PBS) rather than wait for supplies to return to normal.
In 2004, the influenza vaccine was given to 79 per cent of Australians aged over 65 years.
Top of page
Results
Laboratory-confirmed influenza represents a small proportion of all influenza cases in the year and consequently the estimation of the circulating strains is based on a small sample, and should be interpreted with caution. As 2004 was the first time that all the sentinel schemes used the same case definition, this is the first report where comparisons of ILI rates, across all sentinel schemes, with the rates from the previous year, are possible.
National Notifiable Diseases Surveillance System
In 2005, 4,575 laboratory-confirmed cases of influenza were reported to NNDSS, which represents a 115 per cent increase from the number of notifications in 2004 (Figure 1). All jurisdictions reported laboratory-confirmed influenza to NNDSS, although Tasmania reported very few cases due to limited access to laboratory testing for influenza.
Figure 1. Notifications of laboratory-confirmed influenza to the National Notifiable Diseases Surveillance System, Australia, 2003 and 2005, by week of onset and type
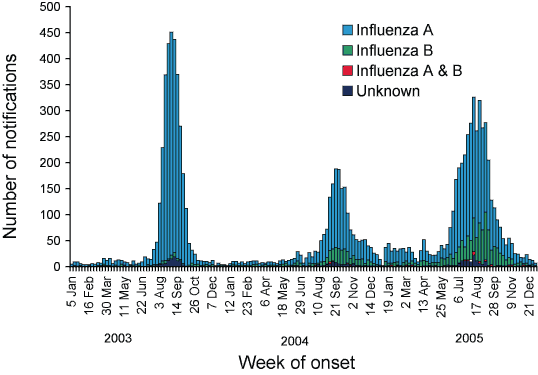
In 2005, influenza A was the predominant type notified (73.1%), while influenza B activity continued to increase compared to previous years (Table 1).
Table 1. Notifications of laboratory-confirmed influenza to the National Notifiable Diseases Surveillance System, Australia, 2003 to 2005, by type
Year |
Proportion of notifications
(%) |
|
|
| |
Influenza A |
Influenza B |
Influenza A & B |
Unknown |
Number of notifications |
| 2005 |
73.1 |
22.8 |
1.4 |
2.7 |
4,575 |
| 2004 |
75.8 |
19.6 |
1.2 |
3.4 |
2,132 |
| 2003 |
93.5 |
3.6 |
0.0 |
2.9 |
3,483 |
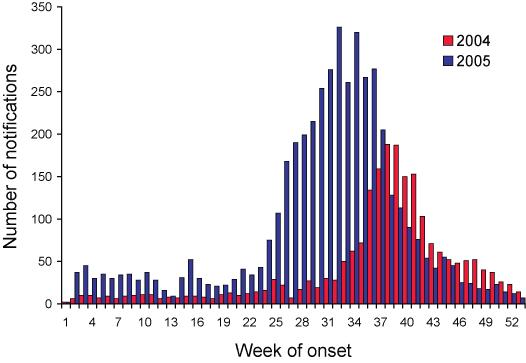
Notifications of laboratory-confirmed influenza started to increase in early June (week 24) and peaked in early August (week 32) (Figure 2). The influenza season started nearly two months earlier than in 2004, and was characterised by a steady rise in the rate of notifications over 10 weeks.
Figure 2. Notifications of laboratory-confirmed influenza to the National Notifiable Diseases Surveillance System, Australia, 2004 and 2005 comparison, by week of onset
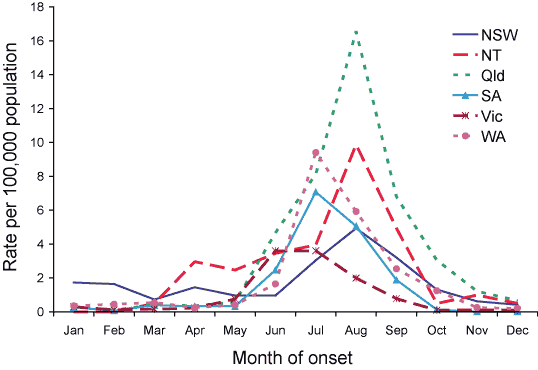
A comparison of notification rates in each jurisdiction (with the exception of the Australian Capital Territory and Tasmania) is shown in Figure 3. In April, a small peak occurred in the influenza notification rate in the Northern Territory (3 cases per 100,000 population). Peak influenza notification rates occurred in July in Victoria (4 cases per 100,000 population), Western Australia (9 cases per 100,000 population), and South Australia (7 cases per 100,000 population) and in August in Queensland (17 cases per 100,000 population), the Northern Territory (10 cases per 100,000 population) and New South Wales (5 cases per 100,000 population).
Figure 3. Notification rates of laboratory confirmed, Australia, 2005, by jurisdiction and month of onset
National age-specific notification rates are shown in Figure 4. The overall male to female ratio was 1:1. The 0–4 year age group had highest notification rate (82 cases per 100,000 population), representing 23 per cent of all notifications.
Within the 0–4 year age group, infants under the age of one composed 42 per cent of the notifications and had the highest notification rate at 154 cases per 100,000 population (Figure 4 insert). Notification rates among the 0–4 year age group were higher in 2005 (82 cases per 100,000 population) compared to 2004 (39 cases per 100,000 population). There was no difference between the notification rate in the 0–4 year age group during 2005 compared to the mean rate over the last four years.
Figure 4. Notification rates of laboratory-confirmed influenza, NNDSS, Australia, 2005, by age and sex
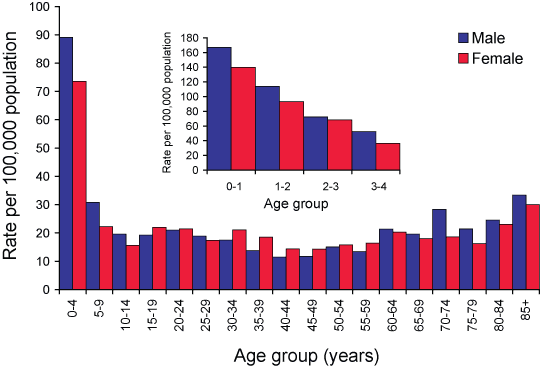
Notification rates amongst persons aged over 65 years were higher in 2005 (22 cases per 100,000 population) compared to 2004 (14 per 100,000 population). The influenza notification rate amongst the over 65 year age group during 2005 was 1.7 times the mean rate over the last four years.
Top of page
Laboratory surveillance
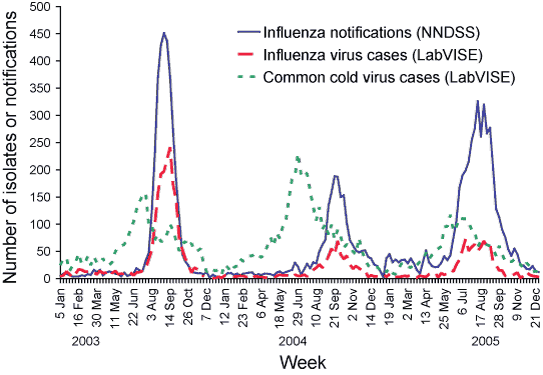
A total of 967 laboratory diagnoses of influenza were reported to LabVISE participating laboratories, which represents a 36 per cent increase from the number of reports in 2004 (Figure 5). In 2005, influenza A was the predominant type reported to LabVISE (73.4%). Reports of influenza to LabVISE started to rise from the middle of June (week 25) a week later than the rise in influenza activity observed in the NNDSS surveillance data. The number of influenza reports to LabVISE reached a plateau from early July until the first week of September.
A further 2,502 laboratory diagnoses of common cold viruses (respiratory syncytial virus, parainfluenza viruses, and rhinoviruses) were reported to LabVISE participating laboratories. Reports of common cold viral isolates to LabVISE started to rise from 13 May (week 15), nine weeks earlier than the rise in influenza activity observed in the NNDSS surveillance data. In 2005, respiratory syncytial virus was the predominant virus reported amongst LabVISE common cold viral isolates (67.0%). Diagnoses of respiratory syncytial virus cases rose from mid-April (week 16) and peaked in mid-June (week 24).
Figure 5. Influenza and common cold virus cases reported to LabVISE compared to notifications of influenza to NNDSS, 2003 to 2005, by week of report
Sentinel general practice surveillance
Australian Sentinel Practice Research Network
The Australian Sentinel Practice Research Network (ASPREN) is a network of general practices, which collect data on ILI. Sentinel general practices contributing to the ASPREN scheme are mostly located in capital cities and larger regional centres on the east coast of Australia. In 2005, an average of 29 general practices reported ILI cases to ASPREN, on an average of 2,996 consultations per week (Table 2). The number of practices reporting to ASPREN and the number of consultations reported on have decreased over the last two years.
Table 2. Number of general practices reporting influenza -like illness to the Australian Sentinel Practice Research Network, 2003 to 2005
Year |
Reporting practices |
Consultations |
| |
Average |
Range |
Average |
Range |
| 2005 |
29 |
15–36 |
2,996 |
1,081–3,698 |
| 2004 |
31 |
11–42 |
3,321 |
1,224–4,219 |
| 2003 |
48 |
32–62 |
4,910 |
2,138–6,587 |
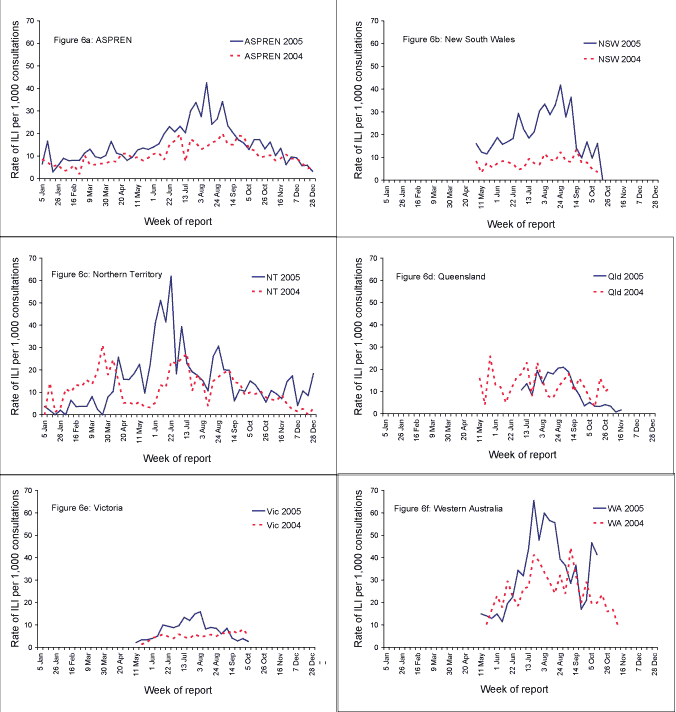
During 2005, a rise in reports of influenza-like illness to ASPREN was evident from mid-June (week 24), one week earlier than in 2004 (Figure 6a). In 2005, the peak ILI rate was observed in early August (week 32) at 42 cases per 1,000 consultations, which was over twice the peak rate in 2004.
State and territory general practice influenza surveillance programs
In New South Wales, ILI surveillance usually runs from May to September. In 2005, the season was extended for a further three weeks due to a small increase in influenza B notifications in the first week of October (Figure 6b). On average, ten general practitioners (range 2–15) reported ILI cases from 5 of 8 Area Health Services (Greater Western, Hunter/New England, Sydney South West, South East Sydney/Illawarra, and Sydney West). These sentinel practitioners reported on an average of 1,178 consultations per week (range 304-1,595). Peaks for ILI were evident in the New South Wales ILI data in early July and late August with peak rates of 33 and 42 ILI per 1,000 consultations respectively (weeks 31 and 34). These peak rates were higher than the 2004 peak rate of 14 per 1,000 consultations, but comparable to the 2003 peak rate of 35 ILI per 1,000 notifications.
Throughout 2005, the Northern Territory Tropical Influenza Surveillance Program received reports of ILI cases from an average of nine sentinel general practices (range 0–14), on an average of 596 consultations per week (range 0–944). The Northern Territory ILI data peaked in mid-June (62 ILI per 1,000 consultations, week 25) with smaller peaks in April and August (Figure 6c). In 2004, peaks were observed in March and July with peak rates of 31 and 27 ILI per 1,000 consultations respectively.
Top of page
In Queensland, an average of 14 sentinel practices provided data per week (range 10–16), including three practices from northern Queensland and 13 practices from central and southern Queensland. The sentinel practices reported on an average of 2,015 consultations per week (range 1,460–2,349). Queensland’s ILI surveillance commenced later in 2005 than in previous seasons due to difficulties recruiting general practitioners. The highest rate (22 ILI per 1,000 consultations) was reported in late August (week 35) (Figure 6d). The peak rate in 2004 was 26 cases of ILI per 1,000 consultations.
Figure 6d. Consultation rates for influenza-like-illness, Queensland Influenza-like Illness Sentinel Surveillance, 2003 to 2005, by week of report
The Victorian Infectious Diseases Reference Laboratory, the WHO Collaborating Centre for Influenza and the Department of Human Services contribute to the Victorian Influenza Surveillance Scheme. On average, 28 general practitioners (range 20–37) were recruited to report ILI rates per 100 consultations per week between May and October. These practitioners reported on an average of 6,282 consultations per week (range 4,066–8,085). In Victoria in 2005, ILI rates started to rise in early June (week 23) and peaked in the first week of August (peak rate 16 cases per 1,000 consultations) (Figure 6e). This was double the peak rate of 2004, reported in late September.
During the 2005 winter season, 20 medical practices were involved in the influenza surveillance program in Western Australia. The majority (16) of the sentinel practices were based in the Perth metropolitan area. Country practices included Kalgoorlie (Goldfields), Busselton (Southwest), Tom Price (Pilbara) and Geraldton (Midwest). On average, 15 practices reported per week (range 10–18) on an average of 2,083 consultations per week (1,029–2,632). In Western Australia, the ILI rate per practice peaked in late July 2005 at 66 cases per 1,000 consultations (Figure 6f). This represents a 48 per cent increase compared to the peak in early September 2004. Influenza-like illness activity started to increase from mid-June 2005 (week 24).
Figure 6. Consultation rates for influenza-like-illness (ILI), ASPREN, 2003 to 2005, by sentinel surveillance scheme and week of report
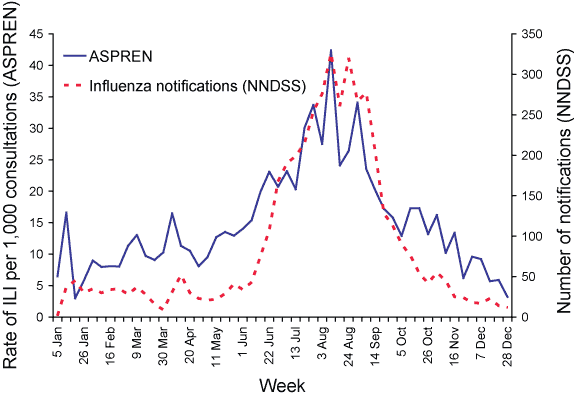
A comparison of ASPREN influenza-like illness reports with influenza notification numbers reported to the NNDSS during 2005 is shown in Figure 7. ILI reports to ASPREN rose in mid-June (week 24) at the same time as the rise in influenza notifications to NNDSS. Furthermore, both surveillance systems peaked in the same week in early August (week 32).
Figure 7. Comparison of reports of influenza-like-illness to ASPREN with notification numbers from NNDSS, Australia, 2005, by week of report
As shown in Table 3, the mid-June (week 24) rise in laboratory-confirmed influenza notifications coincided with the rises observed in most ILI surveillance systems. In this report NNDSS notification data are presented by week of onset (diagnosis date), a reflection of actual disease occurrence in the population. When analysed by date of notification to the NNDSS, a reflection of recognition of disease occurrence by the system, epidemic curves shifted 10 days later. Therefore, three ILI surveillance systems (ASPREN, the Victorian Influenza Surveillance Scheme, and Western Australian sentinel general practices) detected the start of the influenza season by 1 to 2 weeks before NNDSS.
Table 3. Comparison of rises and peaks in influenza surveillance system data, Australia, 2005
Surveillance system |
Week of first rise |
Week of peak |
NNDSS |
| laboratory-confirmed influenza notifications* |
24 |
32 |
LabVISE |
| Influenza reports |
25 |
28 |
| All common cold virus reports |
15 |
23 |
| Respiratory syncytial virus reports |
16 |
24 |
ASPREN |
| Influenza-like illness (ILI) reports |
24 |
32 |
State and territory GP ILI surveillance |
| New South Wales |
26 |
34 |
| Northern Territory |
15 |
25 |
| Queensland |
30 |
35 |
| Victoria |
23 |
31 |
| Western Australia |
24 |
29 |
| Australia Post absenteeism data |
20 |
24 |
Top of page
Absenteeism surveillance
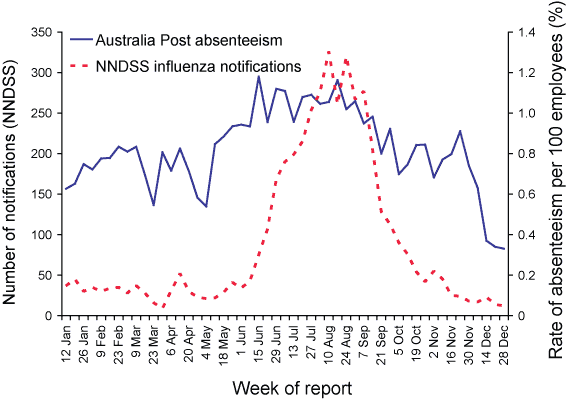
Absenteeism surveillance is a non-specific index of influenza activity. In 2005, national absenteeism rates peaked in mid-June (week 24) at 1.2 per cent (n=389); an increase of 44 per cent from the average of 0.8 per cent absentees per week (n=271) during the reporting period. The absenteeism rates began to rise from week 20 (mid-May) and peaked on week 24, preceding the rise and peak influenza notification activity in NNDSS by four and eight weeks respectively (Figure 8).
Figure 8. Rates of absenteeism and notification rates of influenza, Australia, 2005, by week of report
WHO Collaborating Centre for Reference and Research on Influenza
The WHO Collaborating Centre for Reference and Research on Influenza received 1,174 isolates or clinical specimens from Australian laboratories in 2005 that yielded viable influenza viruses (second only to 2002 for total number of isolates received). These were all analysed antigenically using the haemagglutination inhibition assay which identified 689 (58.7%) as A(H3N2) strains, 210 (17.9%) as A(H1N1) strains and 275 (23.4%) as influenza B strains. Sequence analysis of the variable (HA1) region of the haemagglutinin gene was undertaken for 76 strains (41 H3, 16 H1, 29 B) and of the neuraminidase gene for 21 strains (12 H3, 4 H1, 5 B). The 2005 Australian A(H3) viruses were mostly antigenically similar to the 2005 vaccine strain A/Wellington/1/2004 but a proportion of viruses had a reactivity pattern closer to A/California/7/2004-like viruses and a significant proportion of viruses did not match either of these patterns (Table 4).
Genetic analysis of the Australian A(H3) 2005 isolates (Figure 9) showed that most viruses fell into one of three subgroups based on the HA1 domain of the haemagglutinin gene. One group were similar to A/Victoria/533/2005, another were similar to A/California/7/2004 and a third group were similar to A/Darwin/1/2005 (with the latter group increasing in proportion towards the end of the 2005 season).
Figure 9. Evolutionary relationships between influenza A(H3) haemagglutinins (HA1 region)
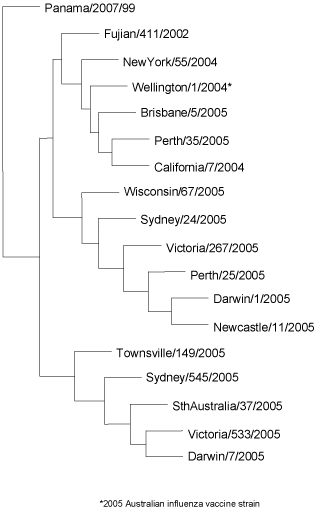
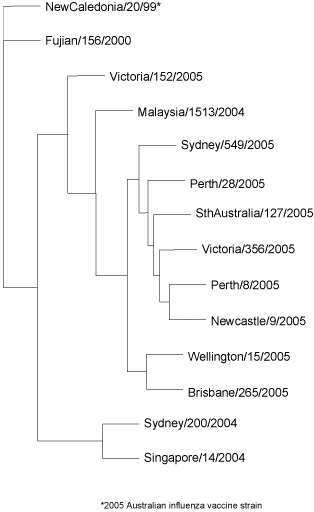
All of the 210 A(H1) isolates had an N1 type neuraminidase (i.e. A(H1N1)) as determined by an ELISA assay and these mostly remained antigenically close to the reference and vaccine strain A/New Caledonia/20/99. Genetically, most of the A(H1) 2005 Australian viruses fell into one subgroup that was distinguishable from A/New Caledonia/20/99 represented by A/Malaysia/1513/2004 (Figure 10).
Figure 10. Evolutionary relationships between influenza A(H1) haemagglutinins (HA1 region)
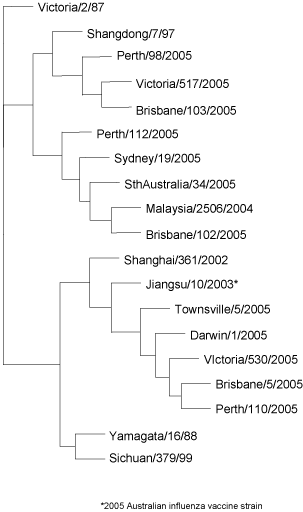
Of the 275 influenza B viruses analysed 51.3 per cent were antigenically and genetically closely related to the reference virus B/Shanghai/361/2002 (B/Yamagata/16/88-lineage) while the remaining 48.7 per cent were B/Hong Kong/330/2001-like or B/Malaysia/2506/2004-like strains (B/Victoria/2/87-lineage) (Figure 11).
Figure 11. Evolutionary relationships between influenza B haemagglutinins (HA1 region)
Consistent with the antigenic drift in the A(H3) isolates demonstrated with ferret antisera (Table 4), serological studies conducted with pre– and post-vaccination human sera from recipients of vaccine containing the A/Wellington/1/2004 strain showed a reduction in antibody titres to some 2005 A(H3) isolates. The Australian 2005 vaccine also contained a B/Shanghai/361/2002-like strain (B/Jiangsu/10/2003) which covered the 2005 influenza B isolates from this lineage fairly well but as expected had reduced titres to 2005 B viruses from the alternative lineage (B/Malaysia/2506/2004) which made up the other half of the isolates analysed. As both lineages have co-circulated in the past three years in Australia it has been difficult to predict which lineage will predominate from year to year and this has made selection of the B component of the vaccine formulation problematic in recent years.
Table 4. Antigenic comparisons of influenza A(H3) viruses by the haemagglutination-inhibition test
Virus antigen |
Ferret antiserum |
| Reciprocal haemagglutination-inhibition titre |
| A/Fujian |
A/Wellington |
A/California |
| A/Fujian/411/2002 |
160 |
640 |
160 |
| A/Wellington/1/2004* |
320 |
1,280 |
1,280 |
| A/California/7/2004 |
640 |
640 |
1,280 |
| A/Brisbane/85/2005 |
40 |
40 |
160 |
| A/Townsville/107/2005 |
320 |
1280 |
160 |
| A/Sth/Aust/98/2005 |
160 |
640 |
80 |
| A/Victoria/216/2005 |
320 |
640 |
640 |
| A/Brisbane/49/2005 |
40 |
80 |
80 |
Top of page
International trends in influenza, 2005
During the period February to September 2005, influenza A(H1N1), A(H3N2) and B viruses circulated in many parts of the world. In the Northern Hemisphere, influenza A(H3N2) viruses predominated and caused most outbreaks including a severe and long outbreak in China. Influenza A(H1N1) viruses caused outbreaks in Eastern Europe and Asia between February and April. Influenza B viruses caused outbreaks in several countries in Africa, Asia and eastern Europe.
In the Southern Hemisphere, influenza activity began in April and increased during May in Oceania, and during June in South America. In Oceania and South America, influenza A(H3N2) and B viruses co-circulated and caused several outbreaks including an epidemic of influenza B in New Zealand. Influenza A(H1N1) also circulated at low levels in some countries.
The WHO annual consultation on the composition of influenza vaccines for the Southern Hemisphere, 2006 took place in Malta from 10 to 15 September 2005. The recommended composition of influenza virus vaccines for use in the 2006 Southern Hemisphere influenza season was:
— an A/New Caledonia/20/1999(H1N1)-like virus;
— an A/California/7/2004(H3N2)-like virus;
—a B/Malaysia/2506/2004-like virus.
This recommendation included two changes to the previous Southern Hemisphere vaccine for the 2005 influenza season, with the addition of a new A(H3) virus (replacing A/Wellington/1/2004 with A/California/7/2004 – the actual vaccine strain used will be A/New York/55/2004) and a new B virus (replacing B/Jiangsu/10/2003, a B/Yamagata-lineage virus with B/Malaysia/2506/2004, a B/Victoria lineage virus).
There were widespread outbreaks of A(H5N1) HPAI influenza in chickens, ducks and other birds throughout South East Asia in 2005. According to the official WHO figures 95 human infections with H5N1 occurred in five countries resulting in 41 deaths. No H5N1 infections were detected in birds or humans in Australia in 2005.
Whilst the temporal pattern of influenza in New Zealand is broadly similar to that in Australia, outbreaks often begin earlier. The New Zealand consultation rates for ILI started to increase in mid-May, a few weeks earlier than in 2003, and peaked in week 27 in late July. During the 2005 winter, New Zealand experienced large outbreaks of influenza type B in school-aged children. This epidemic was associated with significant morbidity with some schools in Auckland, and Wellington experiencing student absenteeism rates in excess of 20 per cent. During this epidemic, three children died suddenly from complications of infection with influenza B/Hong Kong/330/2001 (B/Victoria/2/87-lineage). According to WHO recommendations a B/Yamagata/16/88-lineage strain was included in the 2005 vaccine for Australia and New Zealand, in place of a B/Hong Kong/331/2001-like strain.
The WHO Influenza Centre typed 770 isolates from New Zealand in 2005. Of these the majority were B strains (87.6%) and were mainly of the B/Victoria/2/87-lineage (80%) with a minority (20%) being of the B/Yamagata/16/88-lineage. Of the remaining viruses analysed, 72 were A(H3) (9.2%) and 14 A(H1) (0.2%). Overall, influenza activity in New Zealand in 2005 was average and similar to 2003 but above the levels seen in the 2004 season (a full report on the 2005 influenza season in New Zealand is available from: http://www.surv.esr.cri.nz/PDF_surveillance/Virology/FluAnnRpt/InfluenzaAnn2005.pdf).
Top of page
Discussion
The 2005 influenza season in Australia started earlier and was substantially larger than in 2004. Compared with recent seasons, the 2005 influenza activity was higher than normal, relatively early, and sustained in that it lasted for around four months. Influenza A was again the predominant type isolated in Australia, however, the proportion of diagnoses of influenza B increased for the third successive year.
The rise in reports of common cold viruses to LabVISE preceded the start of the influenza season by nine weeks. Respiratory syncytial virus was the predominant viral isolate amongst these common cold virus reports, and the rise in RSV reports preceded the influenza season by eight weeks. The LabVISE common cold data coincided with the rise observed in absenteeism rates during 2005 reported by Australia Post.
The majority of the Australian isolates (58.7%) analysed at the WHO Influenza Centre were A(H3N2) strains and a significant number of strains showed a degree of heterogeneity based on their antigenic and genetic characteristics. Many strains were antigenically closely matched with A/Wellington/1/2004 while others appeared to be more closely matched to A/California/7/2004-like viruses and a third group reacted poorly with reagents prepared against both of these viral lineages. These sub-groupings could also be seen from the haemagglutinin gene sequence analysis and an increasing proportion of low-reacting strains had the characteristic amino acid changes in the HA1 gene of S193F and D225N from other strains.
In Australia, like many other countries in 2005, the two lineages of influenza B viruses (B/Victoria/2/87 and B/Yamagata/16/88-) co-circulated. An almost even proportion of the two lineages circulated in Australia unlike in New Zealand where approximately 80 per cent of all B strains isolated were of the B/Victoria-lineage.
Influenza A(H1) viruses began to re-emerge in Australia in 2005 after very few viruses were isolated in the previous three years, and made up some 17.8 per cent of total influenza viruses. Interestingly while some genetic changes were evident in the circulating A(H1) strains, little change was seen in their antigenic characteristics when compared to the reference and vaccine A(H1N1) strain A/New Caledonia/20/99.
During 2005, notable influenza outbreaks occurred in New South Wales. Four of the New South Wales outbreaks were reported from aged care facilities and one outbreak from a school. Influenza B was isolated from patients in the first outbreak (in July) and influenza A in the remaining four outbreaks that occurred between August and September. Antiviral drugs (Oseltamvir) were used to control the outbreaks in the aged care facilities, and appeared to be successful. No deaths associated with these outbreaks were reported.
The rate of notifications amongst persons aged over 65 years was the highest recorded in the last five years. The vaccine coverage in this age group in 2005 was unknown. Limited influenza vaccine supply during 2005 may explain the higher than expected notification rates amongst this age group. Amongst vaccinated over 65-year-olds, vaccine effectiveness was expected to be high with regard to circulating influenza A strains, but less so for circulating influenza B strains. However, this age group had a lower proportion of influenza type B infections (18.8%) compared to the rest of the population (24.2%). Vaccine effectiveness may only explain a small proportion of the observed increase in influenza in the over 65 year age group during 2005. When available, the Australian Institute of Health and Welfare data on deaths due to influenza and pneumonia amongst the over 65 year age group during 2005 will clarify whether increased influenza infection rates led to increased mortality.
Preparation for an influenza pandemic is presently a high priority in Australia. Outbreaks of avian influenza ('bird flu') amongst poultry in neighbouring Asian countries, Europe and Africa during 2004 and 2005 have heightened the likelihood of an influenza pandemic occurring. In a pandemic, the priority groups and the timing of vaccination may be quite different from those during inter-pandemic periods. In addition, the number of vaccine doses and the optimal time for vaccination may differ. In June 2005, the National Influenza Pandemic Action Committee (NIPAC) released The Australian Management Plan for Pandemic Influenza (http://www1.health.gov.au/internet/main/publishing.nsf/Content/pandemic%20influenza-1). NIPAC has included guidelines for vaccine use during inter-pandemic and pandemic periods within the management plan (Section 3 and Annex 3), and will advise health authorities regarding priority groups, dosing schedules and timing of vaccination should a pandemic occur. The Australian Management Plan for Pandemic Influenza is expected to be revised during 2006.
Top of page
Online references for influenza :
Australian Government Department of Health and Ageing
National Influenza Surveillance Scheme: http://www1.health.gov.au/internet/main/publishing.nsf/Content/Influenza-1
Pandemic influenza information: http://www1.health.gov.au/internet/main/publishing.nsf/Content/pandemic%20influenza-1
Influenza-like illness surveillance in Australia
NSW Health influenza surveillance report (May to October): http://www.health.nsw.gov.au/infect/diseases.html#I
The Victorian Influenza Surveillance Scheme publishes fortnightly reports (May to October), available at: www.vidrl.org.au
South Australian information for general practitioners on influenza and RSV diagnoses: http://www.dh.sa.gov.au/pehs/notifiable-diseases-summary/flu-resp-intro.htm
Influenza data from New Zealand compiled by ESR Kenepuru Science Centre: http://www.surv.esr.cri.nz/virology/influenza_weekly_update.php
Additional Australian influenza data and information can be accessed at:
Melbourne WHO Collaborating Centre for Influenza: http://www.influenzacentre.org
CSL Limited influenza information website: http://www.flu.com.au/
National Institute of Clinical Sciences (NICS) influenza website: http://www.fightflu.com.au/
Top of page
Acknowledgement
The authors would like to thank all contributors for the time they have invested in the collection of these data. They include: the Australian Sentinel Practice Research Network; the Virology and Serology Laboratory Reporting Scheme reporting laboratories; the New South Wales Department of Health; the Victorian Department of Human Services; the Northern Territory Department of Health and Community Services; Queensland Health; the Western Australian Department of Health; Australia Post; and the World Health Organization Collaborating Centre for Reference and Research on Influenza. The authors would also like to thank the national influenza centres, laboratories in Australia, South East Asia, New Zealand and Oceania. The Melbourne WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health and Ageing.
Thanks also to staff of the Surveillance Systems and Policy Section, in particular Stefan Stirzaker, for his support in data management of the NNDSS and supplemental data collections.
Top of page
References
1. Australian Institute of Health and Welfare. State and Territories GRIM (General Record of Incidence of Mortality) Book – Pneumonia and influenza : Australian Institute of Health and Welfare, Canberra; 2005.
2. Manuguerra JC, Hannoun C, Simon F, Villar E, Cabezas JA. Natural infection of dogs by influenza C virus: a serological survey in Spain. New Microbiol 1993;16:367–371.
3. Joosting AC, Head B, Bynoe ML, Tyrrell DA. Production of common colds in human volunteers by influenza C virus. Br Med J 1968;4:153–154.
4. Cox NJ, Tamblyn SE, Tam T. Influenza pandemic planning. Vaccine 2003;21:1801–1803.
5. Potter CW. Chronicle of influenza pandemics. In: Textbook of influenza: Oxford: Blackwell Science; 1998.
6. Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, Fukuda K. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis 1998;178:53–60.
Top of page
Author affiliations
This report was published in Communicable Diseases Intelligence Vol 30 No 2, June 2006.
