The Australian Gonococcal Surveillance Programme monitors the antibiotic susceptibility of Neisseria gonorrhoeae isolated in all States and Territories. In 2005 the in vitro susceptibility of 3,886 isolates of gonococci from public and private sector sources was determined by standardised methods. Different antibiotic susceptibility patterns were again seen in the various jurisdictions and regions. Resistance to the penicillins nationally was 29.5 per cent and, with the exception of the Northern Territory, ranged between 14 and 47 per cent. Quinolone resistance in gonococci increased with resistance to this agent found in all jurisdictions and in a larger proportion of strains and with higher MICs. Nationally, 29 per cent of all isolates were ciprofloxacin-resistant and most of this resistance was at high minimal inhibitory concentration (MIC) levels. All isolates remained sensitive to spectinomycin. Slightly more than one per cent of isolates showed some decreased susceptibility to ceftriaxone (MIC 0.06 mg/L or more). A high proportion of gonococci examined in larger urban centres were from male patients and rectal and pharyngeal isolates were common. In other centres and in rural Australia the male to female ratio of cases was lower, and most isolates were from the genital tract. Commun Dis Intell 2006;30:205–210.
Top of page
Introduction
Antimicrobial resistance (AMR) surveillance in Neisseria gonorrhoeae has been undertaken by the Australian Gonococcal Surveillance Programme (AGSP) continuously since 1981 to provide data regarding the most reliable therapy for individual and programmatic treatment of gonococcal disease.1,2 There has been a continuing and increasing problem of AMR in gonococci in Australia,2,3 and this has compromised disease control efforts and necessitated changes to use of more expensive and/or injectable antibiotics. Standardised treatment regimens for gonorrhoea utilise single dose treatments that seek to cure 95 per cent or more of cases.4 Where treatment with oral agents such as the penicillins and quinolones is retained, continuous monitoring is required to ensure their ongoing effectiveness. There are also increasing numbers of reports of gonococcal isolates showing resistance to multiple antibiotics including decreased susceptibility to the third generation cephalosporin ceftriaxone which is used extensively in Australia.3,5–9 This analysis of AMR in N. gonorrhoeae in Australia is derived from data generated by the AGSP during the 2005 calendar year.
Top of page
Methods
Ongoing monitoring of AMR in gonococci in Australia is performed by the AGSP through a collaborative program conducted by reference laboratories in each state and territory. The AGSP is a component of the National Neisseria Network of Australia and comprises participating laboratories in each state and territory (laboratories are listed in the acknowledgements). This collaborative network of laboratories obtains isolates for examination from as wide a section of the community as possible and both public and private sector laboratories refer isolates to regional testing centres. The increasing use of non-culture based methods of diagnosis has the potential to reduce the size of the sample of isolates available for testing. Details of the numbers of organisms examined are thus provided in order to indicate the AGSP sample size.
Gonococci isolated in and referred to the participating laboratories, were examined for antibiotic susceptibility to the penicillins, quinolones, spectinomycin and third generation cephalosporins and for high-level resistance to the tetracyclines by a standardised methodology.1,10 The AGSP also conducted a program-specific quality assurance (QA) program.11 Antibiotic sensitivity data were submitted quarterly to a coordinating laboratory which collated the results and also conducted the QA program. Additionally, the AGSP received data on the sex of the patient and site of isolation of gonococcal strains. Where available, data on the geographic source of acquisition of antibiotic-resistant isolates were included in the analyses.
Top of page
Results
Number of isolates
There were 3,980 gonococcal isolates referred to, or else isolated in AGSP laboratories in 2005, about nine per cent more than the 3,640 examined in 2004. The source and site of infection of these isolates are shown in Table 1. One thousand two hundred and eighteen gonococci (30.6% of the Australian total) were isolated in New South Wales, 837 (21%) in Victoria, 651 (16.4%) in Queensland, 646 (16.2%) in the Northern Territory, 403 (10%) in Western Australia, and 180 (4.5%) in South Australia with small numbers in Tasmania (23) and the Australian Capital Territory (22). Three thousand eight hundred and eighty-six isolates remained viable for susceptibility testing.
Table 1. Source and number of gonococcal isolates, Australia, 2005, by sex, site and state or territory
| |
|
State or territory |
|
Gender |
Site |
NSW |
NT |
Qld |
SA |
Vic |
WA |
Aust |
| Male |
Urethra |
665 |
379 |
441 |
90 |
569 |
302 |
2,476 |
| |
Rectal |
238 |
4 |
41 |
24 |
105 |
12 |
429 |
| |
Pharynx |
171 |
1 |
15 |
13 |
70 |
6 |
282 |
| |
Other/NS |
48 |
26 |
14 |
0 |
8 |
3 |
101 |
| |
Total |
1,122 |
410 |
511 |
127 |
752 |
323 |
3,288 |
| Female |
Cervix |
90 |
219 |
133 |
51 |
75 |
72 |
642 |
| |
Other/NS |
5 |
15 |
7 |
1 |
10 |
8 |
46 |
| |
Total |
95 |
234 |
140 |
52 |
85 |
80 |
688 |
| Unknown |
Total |
1 |
2 |
0 |
1 |
0 |
0 |
4 |
| Total* |
|
1,218 |
646 |
651 |
180 |
837 |
403 |
3,980 |
The increase in numbers of gonococci nationally (340, 9%) was mainly the result of more isolates from the Northern Territory (increased by 131), New South Wales (105) and Western Australia (73) with smaller increases in Queensland and South Australia with a slight decrease in numbers in Victoria. Numbers in Tasmania and the Australian Capital Territory, although small, approximated those obtained in 2004.
Top of page
Source of isolates
There were 3,288 strains from men and 688 from women, with a male to female (M:F) ratio of 4.7:1, less than the 5.5:1 ratio for 2004. The number of strains from men increased by 111 and there were 129 more from women. Although lower than in 2004, the M:F ratio was again high in New South Wales (11.6:1) and Victoria (8.8:1) where strains were more often obtained from urban populations. The lower ratios in Queensland (3.6:1) Western Australia (4:1) and the Northern Territory (1.7:1) reflected the large non-urban component of gonococcal disease in those regions. Male rectal and pharyngeal isolates were most frequently found in Victoria (23% of isolates from men), New South Wales (36%) and South Australia (29%). These percentages are higher than those recorded in New South Wales and South Australia in 2004 and lower in Victoria, but also may reflect clinical sampling practices in those States. About 3.6 per cent of isolates are shown as being isolated from ‘other’ or unknown sites. These included nine cases of disseminated gonococcal infection in men (0.3%) and 10 (1.5%) in women. Although not all infected sites were identified, isolates from urine samples were regarded as genital tract isolates. Most of the other unidentified isolates were probably from this source, although they were not so specified. There were a small number of isolates from the eyes (10) of both newborn and older infants and also adults, and from Bartholin’s abscesses in women.
Antibiotic susceptibility patterns
In 2005 the AGSP reference laboratories examined 3,886 gonococcal isolates for sensitivity to penicillin (representing this group of antibiotics), ceftriaxone (representing later generation cephalosporins), ciprofloxacin (representing quinolone antibiotics) and spectinomycin and for high level resistance to tetracycline (TRNG). As in past years the patterns of gonococcal antibiotic susceptibility differed between the various states and territories. For this reason data are presented by region as well as aggregated for Australia as a whole.
Penicillins
The categorisation of gonococci isolated in Australia in 2005 by penicillin minimal inhibitory concentration (MIC) is shown in Figure 1. Infections unlikely to respond to the penicillin group of antibiotics (penicillin, ampicillin, amoxycillin, with or without clavulanic acid) are those caused by gonococci shown as ‘penicillinase-producing’ N. gonorrhoeae (PPNG) and ‘RR – relatively resistant’. Resistance in the PPNG group results from the production of beta-lactamase and in those ‘relatively resistant’ by the aggregation of chromosomally-controlled resistance mechanisms (CMRNG).12 Chromosomal resistance is defined by an MIC to penicillin of 1 mg/L or more.1,10 (The minimal inhibitory concentration in mg/L is the least amount of antibiotic which inhibits in vitro growth under defined conditions.) Infections with gonococci classified as fully sensitive (FS, MIC ≤ 0.03 mg/L), or less sensitive (LS, MIC 0.06–0.5 mg/L) would be expected to respond to standard penicillin treatments, although response to treatment may vary at different anatomical sites.
Figure 1. Penicillin resistance of gonococcal isolates, Australia, 2005, by state or territory
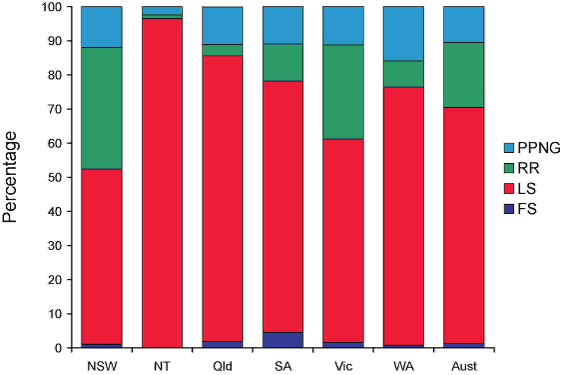
Nationally, 1,148 (29.5%) gonococci were penicillin-resistant by one or more mechanisms in 2005, an increase on the 770 (21.7%) resistant to this group of antibiotics in 2004. Of these, 738 (19%) were CMRNG, almost twice as many as in 2004 (377, 10.6%). This increase was accounted for mainly by an increase in CMRNG in New South Wales from 130 in 2004 to 432 in 2005. Nationally, another 410 (10.5%) were PPNG, the number and proportion of which was little different from 2004 (393, 11.1%). In 2004, there had been more PPNG than in 2003 while numbers of CMRNG had remained unchanged in that period.
Top of page
Penicillin-resistant gonococci were particularly high as a proportion of gonococcal isolates in New South Wales (47.6%; PPNG 12%, CMRNG 35.6%), Victoria (38.6%; PPNG 11.2%, CMRNG 27.6%), Western Australia (23.6%; PPNG 15.9%, CMRNG 7.7%), and South Australia (21.8%, with equal proportions of PPNG and CMRNG. In Queensland, penicillin resistance was also high at 14.4 per cent with 11.1 per cent PPNG. Seven PPNG and one CMRMG were identified in the Australian Capital Territory and in Tasmania there was one PPNG and one CMRNG. In the Northern Territory there were 15 PPNG and six CMRNG resulting in 3.4 per cent of strains that were penicillin resistant (4.2% in 2004). Data on acquisition were available for 128 (31%) infections with PPNG. Sixty-six infections with PPNG were acquired locally and 62 by overseas contact. These contacts were principally in Western Pacific or South East Asian countries including China, Fiji, India, Indonesia (Bali), Malaysia, the Philippines, Thailand, and Vietnam but also through contact in Europe (Germany, Italy, Spain and the United Kingdom), and South Africa.
Ceftriaxone
From 2001 onwards, low numbers of isolates with slightly raised ceftriaxone MICs have been found in Australia. In 2002, there were 21 gonococci with ceftriaxone MICs more than 0.03 mg/L isolated nationally, 10 in 2003 and 24 (0.7%) in 2004. In 2005, there were 48 (1.2%) gonococci with ceftriaxone MICs in the range 0.06 to 0.25 mg/L. Thirty-seven of these were present in New South Wales (3% of isolates there), six (0.7%) in Victoria, and five (0.8%) in Queensland. Forty-five of these 48 gonococci also displayed high-level quinolone resistance and, with the exceptions of one penicillin sensitive isolate and two PPNG, all were also CMRNG.
Spectinomycin
All isolates were again susceptible to this injectable antibiotic.
Quinolone antibiotics
Figure 2 shows the distribution of gonococci with altered susceptibility to quinolones nationally and by state or territory. Thus far resistance to the quinolone antibiotics in N. gonorrhoeae is mediated only by chromosomal mechanisms so that incremental increases in MICs are observed. The AGSP uses ciprofloxacin as the representative quinolone and defines altered resistance as an MIC of 0.06 mg/L or more.10 Treatment with currently recommended doses of 500 mg of ciprofloxacin is effective for strains with a lower level of resistance, viz. 0.06–0.5 mg/L, in about 90 per cent of cases, but lower doses of the antibiotic will result in treatment failure more often. At higher levels of resistance i.e. an MIC of 1 mg/L or more, rates of failed treatment rise rapidly. Currently, gonococci with MICs up to 16 and 32 mg/L are being seen in Australia. At MIC levels of 4 mg/L or more treatment failure, even with higher ciprofloxacin doses, approaches 100 per cent.
Figure 2. Percentage of gonococcal isolates which were less sensitive to ciprofloxacin or with higher level ciprofloxacin resistance and all strains with altered quinolone susceptibility, Australia, 2005, by state or territory
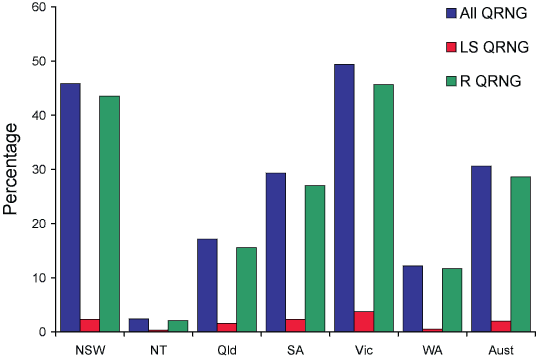
Nationally in 2005, 1,190 (30.6%) gonococci had some level of resistance to quinolones (QRNG), a further increase over the 825 (23.3%) QRNG detected throughout Australia in 2004. In 2003, a total of 529 (14.4%) were QRNG and in 2002 there were 389 (10%) QRNG. Most of the QRNG (1,113, 93.5%) had resistance at a higher level i.e. MICs ≥ 1mg/L. A similar proportion had higher-level resistance in 2004.
The highest proportion of QRNG was seen in Victoria where the 411 QRNG were 49.4 per cent of the total number examined. This was a further substantial increase in both the number (309) and proportion (36%) of QRNG seen in Victoria in 2004. There was also a considerable increase in QRNG in New South Wales where 555 (45.8%) QRNG were detected in 2005 compared to 331 (30%) in 2004. There were 51 (29.3%) QRNG detected in South Australia (36, 24% in 2004) and 108 (17.1%) in Queensland (103, 16.7% in 2004). In Western Australia, QRNG numbers increased slightly to 46 (12.2%) from the 30 (9.4%) in the previous year, and in other jurisdictions the numbers of QRNG remained low (Northern Territory 15; Tasmania 1; Australian Capital Territory 3).
Top of page
Information on acquisition of QRNG was available in 377 of the 1,190 cases (31%). Three hundred and eighteen of these (84%) were acquired locally and 59 (16%) overseas from sources referred to under PPNG acquisition with contacts also reported in Canada, Hong Kong, Korea and the United States of America.
High-level tetracycline resistance
The spread of high-level tetracycline resistance in N. gonorrhoeae is examined as an epidemiological marker even though tetracyclines are not a recommended treatment for gonorrhoea. There was an upsurge in TRNG isolation in 2002 when 11.4 per cent of strains of this type were detected nationally with little further change in 2003. A further increase in TRNG numbers to 490 in 2004 saw them represent 13.8 per cent of all gonococci. This proportion was unchanged in 2005 when 534 TRNG were detected.
TRNG were present in all state and territories with the highest proportion in Victoria (201 TRNG, 24.2%) and Western Australia (78, 20.7%). Lower proportions of TRNG were present in South Australia (21, 12%), Queensland (74, 11.7%) and New South Wales (138, 11.4%). Lower numbers were found in the Northern Territory (17), Tasmania (4) and the Australian Capital Territory (1).
Top of page
Discussion
Further notable and important changes in the susceptibility of N. gonorrhoeae to antibiotics used for the treatment of gonorrhoea occurred in 2005.
Penicillin resistance is of historic interest only in many parts of Australia, but is of considerable relevance in some non-urban settings with high disease rates where antibiotics of this group remain a cheap, effective and acceptable treatment. Of note was the significant increase in resistance mediated by chromosomal mechanisms in New South Wales where both there and in Victoria CMRNG contributed markedly to the historically high levels of penicillin resistance recorded in this report. In other jurisdictions PPNG were a more prominent cause of penicillin resistance. Although incomplete, data on acquisition of PPNG recorded in 2005 show the continuing influence of resistant gonococci introduced into Australia from overseas and the wide diversity of the country of origin of these imported resistant strains.
Similarly, use of the quinolone group of antibiotics has been discontinued for the treatment of gonorrhoea in many parts of Australia for quite some time because of high levels of resistance. Quinolone resistance too is concentrated mainly in larger urban areas. Almost half of the isolates from Victoria and New South Wales and about a third of all gonococci nationally were resistant to ciprofloxacin and at MIC levels that have also increased substantially in recent years. Again however there were considerable jurisdictional differences in rates of quinolone resistance with the Northern Territory having a low (2.4%) proportion of quinolone resistant gonococci. Of particular relevance is the high rate of sustained endemic transmission of QRNG within Australia. QRNG are also widely distributed in countries close to Australia9 and antibiotics other than quinolones should be used for gonococcal infection acquired outside the country.
The number of gonococci with decreased susceptibility to ceftriaxone increased again in 2005. Although the number of these isolates remains low at about one per cent of all isolates tested, these strains almost always also exhibit high-level resistance to quinolones and penicillins. These findings are consistent with recent Japanese data that suggests that these strains are increasingly prevalent there, are multi-resistant and on occasion are associated with treatment failure with oral third generation agents not available in this country.5,6,8 Ceftriaxone is the third generation cephalosporin most used for treatment of gonorrhoea in Australia. The recommended dose of ceftriaxone for uncomplicated mucosal infection in Australia is 250 mg intramuscularly, higher than that in some other treatment schedules in place overseas. This local recommendation for a higher dose treatment would appear to be prudent given the increase in MICs to third generation cephalosporins observed in gonococci in Australia and in nearby countries. It is emphasised that to date there has been no instance of failure with ceftriaxone treatment attributable to decreased susceptibility described in Australia. All gonococci tested in Australia in 2005, including those with altered cephalosporin susceptibility, were susceptible to spectinomycin.
Surveillance of antimicrobial resistance is an essential component of local, regional and international efforts for control of gonorrhoea. Standard treatment guidelines can be reliably based on the results of properly conducted surveillance of antimicrobial resistance, but this in turn requires that the data derived be from epidemiologically as well as microbiologically sound monitoring systems.13 As a guide to interpretation of AGSP data, the WHO currently recommends that once resistance to an antibiotic has reached a level of 5 per cent in a population, continuing use of that agent should be reconsidered. A continuing commitment to maintenance of culture-based systems is still required to examine gonococci in sufficient numbers to detect resistance rates at the 5 per cent level and the sample should also be as representative as possible.14 Despite the increasing use of non-culture based methods for the diagnosis of gonorrhoea, the number of gonococcal isolates available for testing in Australia under the AGSP remains satisfactory for surveillance purposes.
Top of page
Acknowledgements
The AGSP thanks the Australian Government Department of Health and Ageing for continued financial support and the many laboratories, private and public, throughout Australia for submission of isolates for testing.
Members of the Australian Gonococcal Surveillance Programme in 2005 (and to whom isolates should be referred) were: John Bates, Denise Murphy and Vicki Hicks, (Queensland Health Scientific Services, Coopers Plains, Queensland); Athena Limnios, Sanghamitra Ray, Nhu Lan Nguyen and John Tapsall, (Department of Microbiology, The Prince of Wales Hospital, Randwick, New South Wales); Julia Griffith, Mark Veitch, and Geoff Hogg, (The Microbiological Diagnostic Unit (PHL), Department of Microbiology and Immunology, University of Melbourne, Parkville, Victoria); Ann Weaver, (Infectious Diseases Laboratories, Institute of Medical and Veterinary Science, Adelaide, South Australia); Julie Pearson, (Department of Microbiology and Infectious Diseases, PathWest Laboratory Medicine, Royal Perth Hospital, Western Australia.); Mark Gardam and Alistair Macgregor, (Department of Microbiology and Infectious Diseases, Royal Hobart Hospital, Hobart, Tasmania); Gary Lum and Microbiology Staff, (Microbiology Laboratory, Royal Darwin Hospital, Casuarina, Northern Territory;) Susan Bradbury and Peter Collignon, (Microbiology Department, Canberra Hospital, Garran, Australian Capital Territory).
Top of page
References
1. Australian Gonococcal Surveillance Programme. Penicillin sensitivity of gonococci in Australia: the development of an Australian Gonococcal Surveillance Programme. Br J Vener Dis 1984;60:226–230.
2. Tapsall JW. Monitoring antimicrobial resistance for public health action. Commun Dis Intell 2003;27:S70–S74.
3. Australian Gonococcal Surveillance Programme. Annual report of the Australian Gonococcal Surveillance Programme, 2004. Commun Dis Intell 2005;29:136–141.
4. Tapsall J. Antibiotic resistance in Neisseria gonorrhoeae. World Health Organization, Geneva. 2001. WHO/CDS/CSR/DRS/2001.3. Available from: http://www.who.int/csr/drugresist/Antimicrobial_resistance_in_Neisseria_gonorrhoeae.pdf Accessed 7 May 2006.
5. Akasaka S, Muratani T, Inatomi H, Takahasahi K, Matsumoto T. Emergence of cephem- and aztreonam-high-resistant Neisseria gonorrhoeae that does not produce beta-lactamase. J Infect Chemother 2001;7:49–50.
6. Muratani T, Akasaka S, Kobayashi T, Yamada Y, Inatomi H, Takahashi K, et al. Outbreak of cefozopran (penicillin, oral cephems, and aztreonam)-resistant Neisseria gonorrhoeae in Japan. Antimicrob Agent Chemother 2001;45:3603–3606.
7. Wang SA, Lee MV, O’Connor N, Iverson CJ, Ohye RG, Whiticar PM, et al. Multidrug-resistant Neisseria gonorrhoeae with decreased susceptibility to cefixime—Hawaii, 2001. Clin Infect Dis 2003;37:849–852.
8. Ito M, Deguchi T, Mizutani K-S, Yasuda M, Yokoi S, Ito S-I, et al. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in central Japan. Antimicrob Agent Chemother 2005;49:137–143.
9. The WHO Western Pacific Gonococcal Antimicrobial Surveillance Programme. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific Region, 2004. Commun Dis Intell 2006;30:129–132.
10. Tapsall J, and members of the National Neisseria Network of Australia. Antimicrobial testing and applications in the pathogenic Neisseria. In: Merlino J, ed. Antimicrobial susceptibility testing: methods and practices with an Australian perspective. Australian Society for Microbiology, Sydney, 2004. pp 175–188.
11. Australian Gonococcal Surveillance Programme. Use of a quality assurance scheme in a long-term multicentric study of antibiotic susceptibility of Neisseria gonorrhoeae. Genitourin Med 1990;66:437–444.
12. Ropp PA, Hu M, Olesky M, Nicholas RA. Mutations in ponA, the gene encoding penicillin-binding protein1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. Antimicrob Agent Chemother 2002;46:769–777.
13. World Health Organization. Surveillance standards for antimicrobial resistance. World Health Organization, Geneva, 2002. WHO/CDS/CSR/DRS/2001.5. Available from: http://www.who.int/drugresistance/publications/WHO_CDS_CSR_DRS_2001_5/en/index.html Accessed 7 May 2006.
14. Smith DW, Tapsall JW, Lum G. Guidelines for the use and interpretation of nucleic acid detection tests for Neisseria gonorrhoeae in Australia: a position paper on behalf of the Public Health Laboratory Network. Commun Dis Intell 2005;29:358–365.
Top of page
Author affiliations
Corresponding author: Associate Professor John Tapsall, Microbiology Department, SEALS, The Prince of Wales Hospital Randwick NSW 2031. Telephone: +61 2 9382 9079. Facsimile: +61 2 9398 4275. Email: j.tapsall@unsw.edu.au
This report was published in Communicable Diseases Intelligence Vol 30 No 2, June 2006.
