Surveillance of adverse events following immunisation (AEFI) is an integral
component of the management of immunisation programs. In Australia, national
AEFI surveillance data have been collated in the Adverse Drug Reactions Advisory
Committee (ADRAC) database since 2000. AEFIs are notified to ADRAC by state
and territory health departments, health care professionals, vaccine companies
and the public. Two reports summarising national AEFI data have been published
in Commun Dis Intell for January 2000 to September
2002 ,1 and between October 2002 and December 2003.2
This report summarises national AEFI surveillance data for children aged
less than seven years who received vaccines between 1 January and 30 June
2004 and were reported to ADRAC by 30 September 2004. The average annual
population-based AEFI reporting rates were calculated using mid-2003 population
estimates. Reporting rates per 100,000 doses of vaccine were calculated for
seven vaccines that are funded by the National Immunisation Program using
denominator data from the Australian Childhood Immunisation Register (ACIR)
for the period 1 January to 30 June 2004. Reporting rates could not be estimated
for some vaccines due to inadequate denominator data. These include the pneumococcal,
varicella, influenza and monovalent hepatitis B vaccines.
The data reported here are provisional only. It is important to note
that AEFIs are defined as medically important events that are temporally
associated with immunisation but are not necessarily causally associated
with immunisation. Readers are referred to previous reports for a description
of the national AEFI surveillance system, 1 methods
used to analyse the data 1,2 and information regarding
limitations and interpretation of the data.2 Often,
more than one vaccine is listed as suspected of involvement in the reported
adverse event, so the number of vaccines will be greater than the number
of AEFI records analysed.
There were a total of 219 records of adverse events following immunisation
(AEFI) (24.5 per 100,000 population) for children aged less than seven years
where suspected vaccines were administered in the first six months of 2004
to children aged less than seven years. This was a 54 per cent reduction
on the 474 records (53.0 per 100,000 population) for the corresponding six
month period in 2003. Sixty-six percent (n=144) of records were for children
aged 2 to <7 years, with 11 per cent (n=23) for children aged 1 to <2
years and 24 per cent (n=52) for children aged <1 year. The relative contribution
of children aged 1 to <2 years is lower (down from 33%) compared with
the first six months of 2003, while that of children aged 2 to <7 years
is higher (up from 50%). The male to female ratio was 1.3:1.0, and similar
to that seen previously.
Of the 219 records analysed, 19 (8.7%) were defined as having ‘serious’
outcomes (recovery with sequelae, hospitalisation or death), and was similar
to previously reported (9%).2 Two deaths were
reported: neither was thought to be causally related to vaccination. Other
serious or potentially life-threatening AEFIs reported were hypotonic-hyporesponsive
episode (n=4) and seizure (n=1). The most commonly reported reaction categories
were injection site reaction (n=117; 53%) and fever (n=46; 21%).
One or more of the seven vaccines shown in Table were recorded as being
suspected of involvement in the reported adverse event for 205 of the 219
records analysed. The 14 records that listed other suspected vaccines, for
which adequate denominator data are not available, included pneumococcal
(n=8), monovalent hepatitis B (n=8), varicella (n=7) and influenza (n=1)
vaccines.
The AEFI reporting rates per 100,000 vaccine doses, both overall and
for specific vaccines, were generally similar to those for January 2000–September
2002 1 and significantly lower than observed for
the October 2002–December 2003 period 2 (Table).
The largest reductions in reporting rates were for diphtheria-tetanus-pertussis
(acellular) (DTPa) vaccine and meningococcal C conjugate vaccine (MenCCV)
(Table, Figures 1 and 2). The reporting rate for AEFIs with outcomes defined
as ‘serious’ for the seven vaccines declined slightly from 1.2 to 1.0 per
100,000 doses (Table).
Top of page
Table. Reporting rates of adverse events following immunisation (AEFI) per 100,000 vaccine doses,* children aged less
than seven years, ADRAC database, January to June 2004
Suspected vaccine or AEFI category† |
AEFI records‡ (n) |
Vaccine doses* (n) |
Reporting rate per 100,000 doses § |
Difference || |
| Diphtheria-tetanus-pertussis |
122 |
255,758 |
47.7 |
-16.6 |
| Diphtheria-tetanus-pertussis-hepatitis B |
32 |
226,240 |
14.1 |
-4.8 |
| Haemophilus influenzae type b |
44 |
227,364 |
19.4 |
-2.4 |
| Haemophilus influenzae type b-hepatitis B |
8 |
127,501 |
6.3 |
-5.0 |
| Poliovirus (oral or inactivated) |
50 |
478,488 |
10.4 |
-4.4 |
| Measles-mumps-rubella |
78 |
239,256 |
32.6 |
+1.4 |
| Meningococcal C conjugate |
51 |
179,379 |
28.4 |
-10.3 |
| Total† |
205 |
1,749,075 |
11.7 |
-8.1 |
| ‘Certain’ or ‘probable’ causality rating† |
73 |
1,749,075 |
4.2 |
-5.8 |
| ‘Serious’ outcome† |
17 |
1,749,075 |
1.0 |
-0.2 |
Top of page
Figure 1. Reports of injection site reaction following diphtheria-tetanus-pertussis (acellular)
vaccine, ADRAC database, January 2000 to June 2004, by age group and quarter of vaccination
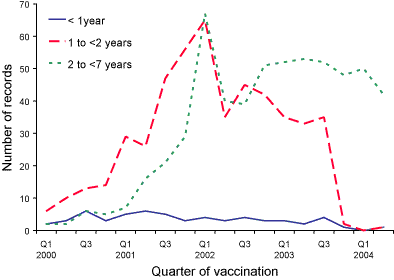
Figure 2. Reports of adverse events reports following meningococcal C conjugate vaccination,
ADRAC database, January 2001 to June 2004, by age group and quarter of vaccination
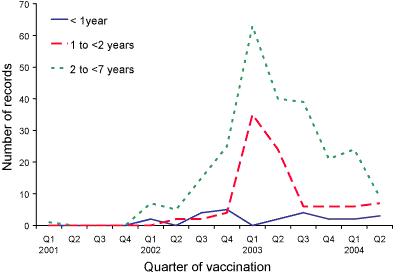
The observed reduction in the number of AEFI reports received for children
aged less than seven years during the first half of 2004, and the change
in the age distribution, corresponds in time with removal of the fourth dose
of DTPa vaccine (previously due at 18 months of age) from the Australian
Standard Vaccination Schedule in September 2003 (Figure 1, Table), and with
the completion of the MenCCV catch-up campaign for children born before 2002
(Figure 2). The lower AEFI reporting rates per 100,000 doses of MenCCV may
also be due to more accurate denominator data recorded on the ACIR and/or
to increased familiarity among providers about the more common, less serious
side-effects of the vaccine resulting in reduced reporting to ADRAC.
Top of page
Conclusion
There was a marked decline in the overall AEFI reporting rates for children
aged less than seven years during January–June 2004 compared with the previous
year. Reporting rates of AEFIs with outcomes defined as ‘serious’ were stable.
Annual reports summarising all AEFI data collated in the ADRAC database are
planned for the future with supplementary reports summarising AEFI data for
children aged less than seven years for vaccines received in first six months
of each year.
Top of page
Acknowledgements
We thank Professor David Isaacs (Adverse Drug Reactions Advisory Committee)
and Professor Peter McIntyre and Doctor Nicholas Wood (NCIRS) for assisting
with aspects of this report.
Top of page
References
1. Lawrence G, Menzies R, Burgess M, McIntyre P, Wood N, Boyd I, et al.
Surveillance of adverse events following immunisation: Australia, 2000–2002.
Commun Dis Intell 2003;27:307–323.
2. Lawrence G, Boyd I,
McIntyre P, Isaacs D. Surveillance of adverse events following immunisation:
Australia 2002–2003. Commun Dis Intell 2004;28:324–38.
Top of page
Author affiliation
1. National Centre for Immunisation Research and Surveillance of Vaccine Preventable
Diseases, University of Sydney and The Children’s Hospital at Westmead, New
South Wales
2. Adverse Drug Reactions Unit, Therapeutic Goods Administration, Canberra, Australian
Capital Territory
Corresponding author: Dr Glenda Lawrence, National Centre for Immunisation
Research and Surveillance of Vaccine Preventable Diseases, University of
Sydney and The Children’s Hospital at Westmead, Locked Bag 4001, Westmead
NSW 2145. Telephone: +61 2 9845 0520. Facsimile: +61 2 9845 3095. Email:glendal@chw.edu.au
This article was published in Communicable Diseases Intelligence Vol 28 No 4, December 2004.
