Please note: Some minor amendments have been made to this article post-printing on 4 May 2005. In particular, Tables 15 and 16 have been updated and the Map replaced.
Paul Roche,1 Vicki Krause,2 Mark Bartlett,3 David Coleman,4 Heather Cook,2 Megan Counahan,5 Craig Davis,6 Letitia Del Fabbro,7 Carolien Giele,8 Robyn Gilmore,3 Riemke Kampen,9 Margaret Young6 with laboratory data supplied by Geoff Hogg,10 Denise Murphy11 and Michael Watson12
Introduction | Methods | Results | Discussion | References
Abstract
There were 2,174 cases of invasive pneumococcal disease (IPD) notified to the National Notifiable Diseases Surveillance System (NNDSS) in Australia in 2003; a rate of 10.9 per 100,000 population. The notification rate varied between states and territories and by geographical region with the highest rates in the north of the country. Invasive pneumococcal disease was reported most frequently in children aged less than two years (98.8 cases per 100,000 population). Enhanced surveillance for IPD in 2003 was carried out in all states and territories, providing additional data on 1,842 (85%) of all notified cases. Rates of IPD in Indigenous Australians were three times the rate in non-Indigenous Australians. There were 125 deaths attributed to IPD resulting in an overall case fatality rate of 6.8 per cent. Seventy-one per cent of all pneumococcal isolates serotyped were serotypes in the seven-valent conjugate vaccine and 91 per cent were serotypes in the 23-valent polysaccharide pneumococcal vaccine. The clinical presentation and risk factors for IPD varied between Indigenous and non-Indigenous cases and non-vaccine serotypes occurred more frequently among Indigenous children and adults. Data from three years of surveillance indicate an early impact of the 7-valent vaccine in the target population. Commun Dis Intell 2004;28:441–454.
Top of page
Introduction
Infection with Streptococcus pneumoniae is a leading cause worldwide of otitis media, pneumonia, bacteraemia, meningitis, responsible for significant morbidity and mortality in infants, the elderly and those with predisposing risk factors. Invasive pneumococcal disease (IPD) is the clinical condition in which S. pneumoniae infects a normally sterile site such as blood, cerebrospinal fluid or pleural fluid. IPD presents most commonly as pneumonia in adults and bacteraemia in children. The risk of disease is highest among people who are immunocompromised or have a chronic illness. IPD disease in Australia is generally a disease of the very young and the very old. The incidence of IPD in Indigenous Australians has been much higher than that of non-Indigenous Australians.
The escalating resistance of the pneumococcus to antibiotics has been an important factor for the development and introduction of new pneumococcal vaccines. In Australia the rate of penicillin resistant pneumococci increased from 1 per cent in 1984 to 25 per cent in 1997.1 Reduced susceptibilities to other antimicrobials has also emerged in recent years with the rate of reduced susceptibility to third generation cephalosporins in Australia reaching 13 per cent in 1997.2 Multi-drug resistant pneumococci have been documented around the world and have been associated with outbreaks of meningitis in infants.3 In 1999, 6.8 per cent of invasive and 16.7 per cent of non-invasive pneumococcal isolates in Australia were multi-drug resistant.4
Ninety serotypes of S. pneumoniae identified by the polysaccharide composition of their capsule have been described. Immunity to pneumococcal infection is thought to be serotype specific. Vaccines containing pneumococcal polysaccharides from a varying number of serotypes have been available for many years, with a 23-valent polysaccharide vaccine (23vPPV) being used in Australia from 1986 (Table 1). Polysaccharide pneumococcal vaccines were shown to be poorly immunogenic in young children.5 A vaccine in which polysaccharides from seven serotypes coupled to a protein carrier (mutated diphtheria toxoid) was developed to provide an effective pneumococcal vaccine for children and in a trial in the USA in infants aged 2 to 15 months of age demonstrated an efficacy of 93.9 per cent. 6 This conjugate vaccine (7vPCV) was licensed for use in Australia in January 2001 and a nationally funded vaccination program for children at high risk commenced in June 2001 (Table 1).
Table 1. Recommendations for pneumococcal vaccination, Australia, 2003
| Vaccine |
23-valent polysaccharide vaccine |
7-valent conjugate vaccine |
| Pneumococcal Serotypes |
1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C,19A, 19F, 20, 22F, 23F, 33F
|
4, 6B, 9V, 14, 18C, 19F, 23F |
| Date implemented |
1998 |
July 2001 |
| Target populations |
All individuals aged 65 years and over
Individuals with asplenia
Immuncompromised patients
Aboriginal and Torres Strait Islander people aged 50 years and over
Immunocompetent individuals with chronic illness including chronic cardiac, renal or pulmonary disease, diabetes and alcohol-related problems |
Tier 1: Indigenous children less than 5 years living in central Australia
Tier 2: Indigenous children aged less than 2 years particularly in rural and remote settings
Tier 3: Indigenous children under 2 years living in other settings
Non-Indigenous children less than 2 years living in Central Australia
Non-Indigenous children with conditions predisposing to pneumococcal infection |
| Data source |
NHMRC Immunisation Handbook7th edition, 2000 |
ATAGI recommendations, 2001 |
IPD was made a notifiable disease in all Australian states and territories in 2001 and surveillance data are reported to the National Notifiable Diseases Surveillance System (NNDSS). Additional enhanced surveillance data on IPD has also been collected since 2001 and annual reports have been published.7,8 In this third report, an analysis of the influence of the 7-valent vaccine on IPD in vaccine eligible children has been performed with respect to overall rates of disease, disease caused by vaccine and non-vaccine serotypes and levels of antimicrobial resistance. Baseline data on IPD in all children and the elderly prior to the introduction of universal child and 65 year and older immunisation programs commencing in January 2005 are presented.
Top of page
Methods and Materials
Case definition
A case of invasive pneumococcal disease was defined as the isolation from or the detection in blood, cerebrospinal fluid (CSF) or other sterile site of Streptococcus pneumoniae.
Data collection
Invasive pneumococcal disease has been a notifiable disease in some Australian states and territories for several years. In 2001, IPD was made notifiable in all states and territories and data are forwarded to the NNDSS. Since this required changes to state and territory public health legislation, the data in 2001 was incomplete in some states and territories, but was complete for all jurisdictions from 2002.
NNDSS data in 2003 comprised core data, which is an agreed national dataset on all cases of all notifiable diseases as well as data specific for IPD. The list of the data fields collected the IPD enhanced data set is shown in Table 2.
Table 2. Invasive pneumococcal disease surveillance data supplied by states and Territories used in this report
Data type |
Data fields |
| Demographic |
Date of Birth;
Age;
Indigenous status: (Aboriginal, Torres Strait Islander, Aboriginal
and Torres Strait Islander, Other, Unknown);
Location (optional);
Postcode
|
| Risk factors |
Premature birth (gestation less than 37 weeks);
Congenital abnormality
Anatomical or congenital asplenia;
Immunocompromised (eg: HIV, lymphoma, transplant, multiple myeloma,
nephrotic syndrome etc.);
Chronic illness (eg: cardiac disease, pulmonary disease, CSF leak,
diabetes);
Other risk factors (variable by State) including chronic suppurative
otitis media, failure to thrive, previous IPD or pneumonia, excessive
alcohol consumption, smoking or smoke exposure.
|
| Clinical data |
Clinical presentation (pneumonia, meningitis, bacteraemia, other, unknown);
Date of onset;
Death due to IPD
|
| Microbiology data |
Specimen collection date;
Date laboratory results issued (report date);
Date notification received;
Specimen type (blood, CSF, pleural fluid, joint fluid, other sterile
site);
Specimen culture positive or S. pneumoniae
detected by nucleic acid tests;
Antibiotic susceptibility (penicillin, cefotaxime/ceftriaxone);
Pneumococcal serotype
|
| Vaccination history |
Source of vaccination history (validated, not validated, information
not collected);
Pneumococcal vaccination dates, number of doses and type of vaccine;
Vaccination status: fully vaccinated for age, partially vaccinated
for age, not vaccinated, unknown
|
Clinical presentation
Clinical presentations were coded as pneumonia, meningitis, bacteraemia, other or unknown. Pneumonia was defined as blood culture or nucleic acid test (NAT) positive for S. pneumoniae with clinical and/or radiological signs of pneumonia. Meningitis was defined as the detection of S. pneumoniae in the cerebrospinal fluid (CSF) and/or blood with supportive clinical findings. Bacteraemia was defined as the detection of S. pneumoniae in blood with no localising signs. 'Other' presentations included detection of S. pneumoniae in pleural, peritoneal or joint fluid. More than one clinical presentation could be recorded for each case.
Vaccination
The consensus definitions of vaccination status, vaccination confirmation and vaccine failure are shown in Table 3.
Table 3. Definitions of vaccination status and vaccine failure used in this report
Category |
Definition* |
| Fully vaccinated (child)
|
Those that have had the required doses for age of 7vPCV (or 23vPPV
if age > 18 months) at least 2 weeks prior to infection. Children
aged less than 7 months analysed on an individual basis.
|
| Fully vaccinated (adult)
|
Those that have had the required doses of 23vPPV at least 2 weeks prior
to infection.
|
| Partially vaccinated (child)
|
Those that have received at least one dose, but not all the recommended
doses of vaccine for age.
|
| Partially vaccinated (adult)
|
Those that have been vaccinated with 23vPPV but the time frame is outside the recommended schedule.
|
| Not vaccinated (child or adult)
|
Those that havenever received a pneumococcal vaccine.
|
| Vaccination confirmation
|
Written confirmation through Australian Childhood Immunisation Register,
State or Territory Immunisation register or health record.
|
| Vaccine failure |
A fully vaccinated person (as per the above criteria) with disease due to a serotype found in the corresponding vaccine.
|
Top of page
Populations under surveillance
There were differences in populations under surveillance between jurisdictions in the collection of enhanced IPD data. The age groups on whom enhanced data was collected in 2003 are shown in Table 4.
NNDSS data for 2003 was analysed by date of disease onset while data in the enhanced data sets was analysed by date of notification.
Table 4. Enhanced Invasive pneumococcal disease surveillance data collection by states and territories in 2003
Age group |
Jurisdictions |
| Under 5 years |
Australian Capital Territory (except vaccination status and risk factors)
Queensland
Victoria (except risk factor information)
South Australia (except risk factor information)
New South Wales (Cases in rural Public Health Units (PHU) and South Western Sydney, Hunter, Illawara, Greater Western Sydney, Wentworth and Southeastern Sydney PHUs) |
| Over 50 years |
Victoria (Indigenous cases and vaccine failures only)
South Australia (Vaccine failures only)
New South Wales (Cases in Rural Public Health Units (PHU) and South Western Sydney, Hunter, Illawara, Greater Western Sydney PHUs) |
| All ages |
North Queensland
Northern Territory
Western Australia
Tasmania |
Data analysis
The notification rates presented in this report were calculated using population data from the Australian Bureau of Statistics (ABS). The Estimated Resident Population (ABS 3201.0) in each state and territory and in Australia as a whole, as at June 30, 2003, was used as the denominator in rate calculations. Estimates of the Indigenous Australia population were based on projections from the 2001 census. The ABS calculated projections based on assumptions about future births, deaths and migrations in the Indigenous population and a 'low' and 'high' estimate were reported. The 'low' estimate has been used in this report, consistent with the reporting of other national communicable diseases.
The significance of differences in proportions was calculated using the Chi-square test with Yates correction using Epi-Info 6.
Top of page
Results
Notifications to NNDSS
There were 2,174 notifications of IPD to the NNDSS in 2003. The numbers of notification and the notification rate per 100,000 population are shown in Table 5.
Table 5. Notifications and notification rate per 100,000 population, Invasive pneumococcal disease, Australia, 2003*
| |
ACT |
NSW |
NT |
Qld |
SA |
Tas |
Vic |
WA |
Australia |
| Notifications |
40 |
784 |
72 |
466 |
176 |
43 |
443 |
150 |
2,174 |
| Rate per 100,000 population |
12.4 |
11.7 |
37.3 |
12.3 |
11.5 |
9.0 |
9.0 |
7.7 |
10.9 |
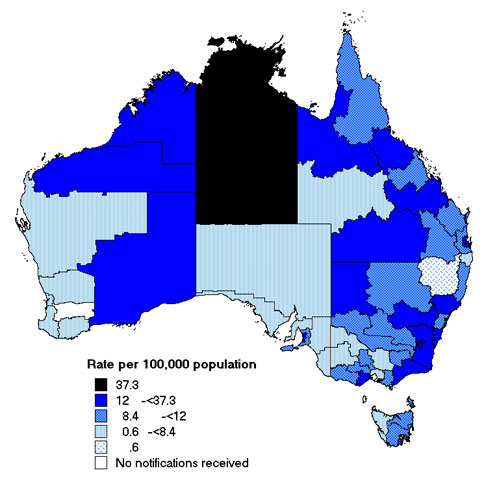
The rates of IPD disease ranged between 7.7 and 12.4 per 100,000 except in the Northern Territory where the rate was 37.3 per 100,000.When the notification rates of IPD were examined by geographical distribution, variation within states was evident (Map).
Map. Notification rates of invasive pneumococcal disease, Australia, 2003 by statistical division of residence
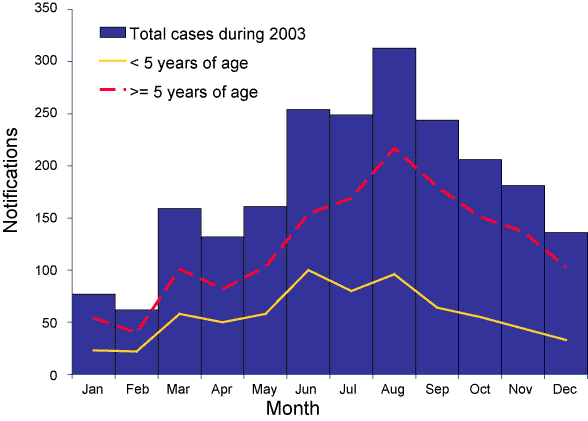
The frequency of cases varied by season with 816 (38%) reported in winter months (June to August). The effect of season was more marked in cases aged five years or more than in younger children (Figure 1).
Top of page
Figure 1. Notifications of invasive pneumococcal disease, by month of report and age group, Australia, 2003
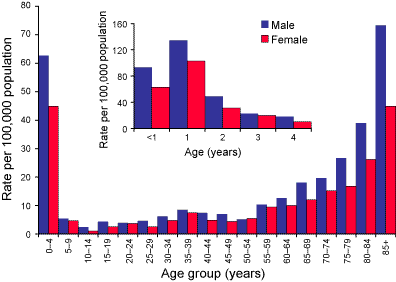
As previously noted, IPD in Australia is largely a disease of the very young and very old. The highest rates of disease were in children aged less than five years and adults aged more than 85 years (Figure 2). Among children aged less than five years, the highest rates were recorded in children aged one year (119 per 100,000 population). There were 488 cases in children aged less than two years of age (98.8 per 100,000) in 2003. In all age groups there were more male than female cases (overall male to female ratio 1.3:1).
Figure 2. Notification rates of invasive pneumococcal disease, by age and sex, Australia, 2003
 Top of page
Top of page
Enhanced IPD surveillance data
Enhanced data were available for 1,842 cases or 85 per cent of notified cases – a similar proportion of cases to that reported on in the 2002 annual report.
Demographics
The demographic characteristics of cases on which enhanced data were collected are shown in Table 6.
Table 6. Demographic profile of Invasive pneumococcal disease cases reported by enhanced surveillance systems, by jurisdiction, Australia, 2003*
| Data |
|
ACT |
NSW |
NT |
Qld |
SA |
Tas |
Vic |
WA |
Total |
| Number |
|
44 |
428 |
72 |
464 |
176 |
43 |
465 |
150 |
1842 |
| Sex |
Male:Female ratio |
1.2:1 |
1.3:1 |
2:1 |
1.3:1 |
1.4:1 |
1.9:1 |
1.4:1 |
1.3:1 |
1.3:1 |
| Age |
<5 years |
11 |
192 |
20 |
163 |
73 |
4 |
131 |
43 |
637 |
| |
5 to 64 years |
20 |
69 |
49 |
200 |
50 |
27 |
181 |
68 |
664 |
| |
>=65 years |
13 |
167 |
3 |
101 |
53 |
12 |
153 |
39 |
541 |
| Indigenous status |
Indigenous |
0
(0%) |
13
(3%) |
51
(71%) |
53
(11%) |
3
(2%) |
0
(0%) |
7
(2%) |
22
(15%) |
149
(8%) |
| |
Non-indigenous |
19
(43%) |
403
(94%) |
21
(29%) |
305
(66%) |
163
(93%) |
39
(91%) |
288 (62%) |
128 (85%) |
1,366 (74%) |
| |
Unknown |
25 (57%) |
12 (3%) |
0 |
106 (23%) |
10 (5%) |
4 (9%) |
170 (36%) |
0 |
327 (18%) |
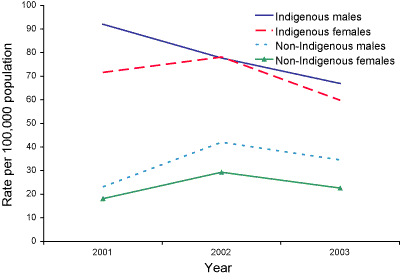
The enhanced data identified 149 cases of IPD among Indigenous people, which represented eight per cent of all cases, a similar proportion to that in 2002. This represented a national rate of 33.5 per 100,000 in Indigenous people compared with the national rate of 10.7 per 100,000. The rates of IPD in Indigenous people were highest in the Northern Territory (87.7 per 100,000) and Queensland (42.6 per 100,000). The national rate of IPD in Indigenous people is likely to be underestimated as incomplete reporting of Indigenous status continues to be a problem in some jurisdictions. Rates in Indigenous children under five years have fallen from 92 to 67 per 100,000 in males and 72 to 60 per 100,000 in females, between 2001 and 2003 respectively, but these represent small declines in total numbers (Figure 3).
Figure 3. Notification rates of invasive pneumococcal disease, Australia 2001 to 2003 in children under 5 years of age, by Indigenous status
Top of page
Clinical presentation
The clinical presentation was reported in 69.8 per cent (1,286/1,842) of cases with enhanced surveillance information (Table 7).
Table 7. Clinical presentations of Invasive pneumococcal disease, by jurisdiction, Australia, 2003
| Clinical presentation* |
ACT |
NSW |
NT |
Qld |
SA |
Tas |
Vic |
WA |
Total |
| Pneumonia(%) |
0
|
208
(48%) |
46
(64%) |
78
(17%) |
48
(27%) |
32
(74%) |
146
(31%) |
104
(69%) |
662
(36%) |
| Meningitis(%) |
1
(2%) |
27
(6%) |
6
(8%) |
19
(4%) |
8
(5%) |
5
(12%) |
32
(7%) |
11
(7%) |
109
(6%) |
| Bacteraemia (%) |
36
(82%) |
180
(42%) |
19
(26%) |
145
(31%) |
87
(49%) |
6
(14%) |
70
(15%) |
49
(32%) |
592
(32%) |
| Other(%) |
3
(7%) |
8
(2%) |
1
(1%) |
10
(2%) |
5
(3%) |
0
|
5
(1%) |
13
(9%) |
45
(2%) |
| Unknown (%) |
4
(9%) |
5
(1%) |
0
|
252
(54%) |
40
(23%) |
0
|
215
(46%) |
0
|
516
(28%) |
Pneumonia was the most common clinical presentation (662 cases, 3.3 per 100,000 population) followed by bacteraemia (592 cases, 2.9 per 100,000 population) and meningitis (109 cases, 0.5 per 100,000 population). Other clinical presentations of IPD accounted for 45 cases. These clinical presentation rates were similar to those reported in 2002.
Clinical presentation varied by age with pneumonia being the most common presentation among cases over 65 years (72%) and bacteraemia the most common presentation among cases in children under five years (68%).
The proportion of IPD cases presenting as pneumonia was significantly higher in Indigenous children (37%) compared with non-Indigenous children (22%). There were no significant differences between Indigenous and non-Indigenous children in the proportions of other clinical presentations (Table 8).
Table 8. Clinical presentations of Invasive pneumococcal disease in Indigenous and non-Indigenous children aged less than 5 years, Australia, 2003
| |
Number of cases (%) |
Indigenous
n=45 |
Non-Indigenous
n=516 |
Significance of difference* |
| Pneumonia |
17 (37%) |
112 (22%) |
p<0.05 |
| Meningitis |
8 (17%) |
54 (10%) |
ns |
| Bacteraemia |
24 (53%) |
329 (64%) |
ns |
| Other |
0 |
20 (4%) |
– |
IPD resulted in 125 deaths in Australia in 2003, a case fatality rate of 6.8 per cent (Table 9). The case fatality rate was significantly higher in cases aged more than 65 years (16.6%) compared with children aged less than five years (1.9%, p<0.001). The case fatality rate was not significantly different in Indigenous cases (4.7%) and non-Indigenous cases (6.9%). There were seven deaths in children aged less than two years of age.
Top of page
Table 9. Case fatality rates for Invasive pneumococcal disease, by jurisdiction, Australia, 2003
| Data |
ACT |
NSW |
NT |
Qld |
SA |
Tas |
Vic |
WA |
Total |
| Cases |
44 |
428 |
72 |
464 |
176 |
43 |
465 |
150 |
1,842 |
| Deaths |
4 |
63 |
4 |
8 |
9 |
6 |
22 |
9 |
125 |
| Case fatality rate (%) |
9% |
14.7% |
5.5% |
1.7% |
5.1% |
13.9% |
4.7% |
6% |
6.8% |
| Deaths in Aged < 5y/total cases aged <5 y (%) |
1/11
9% |
4/192
(2.1%) |
2/20
(10%) |
1/163
(0.6%) |
1/73
(1.4%) |
0/4
(0%) |
2/131
(1.5%) |
1/43
(2.3%) |
12/ 637
(1.9%) |
| Deaths in Aged >65y/total cases aged >65y (%) |
2/13
15% |
54/167
(32.3%) |
0/3
(0%) |
5/101
(5%) |
6/53
(11.3%) |
3/12
(25%) |
15/153
(9.8%) |
5/39
(12.8%) |
90/541
(16.6%) |
| Deaths in Indigenous people /total Indigenous cases (%) |
0/0
(0%) |
2/13
(15.4%) |
2/51
(3.9%) |
2/53
(3.7%) |
0/3
(0%) |
0/0
(0%) |
1/7
(14.3%) |
0/22
(0%) |
7/149
(4.7%) |
| Death in non-Indigenous / total non-Indigenous + 'unknown' cases (%) |
4/44
(9%) |
61/415
(14.7%) |
2/21
(9.5%) |
6/411
(1.4%) |
9/173
(5.2%) |
6/43
(13.9%) |
21/458
(4.6%) |
9/128
(7%) |
118/1693
(6.9%) |
Risk factors for pneumococcal disease
The national surveillance working group defined risk factor categories for IPD. Other risk factors were recorded but varied between jurisdictions. More than one risk factor could be recorded for each case. Recognised risk factors for pneumococcal disease were reported in 640 cases. The most common of these was chronic illness, which included chronic respiratory, cardiac and renal disease. Immunocompromising conditions such as long-term immunosuppressant use were common among IPD cases.
The frequency of risk factors for IPD in Indigenous and non-Indigenous people are shown in Table 10. The rates of chronic illness were significantly higher in Indigenous children aged less than five years with IPD compared with non-Indigenous children in the same age group. Among cases aged five years or more, the proportion of immunocompromised cases was significantly higher among non-Indigenous cases than Indigenous cases.
Table 10. The frequency of risk factors for Invasive pneumococcal disease, by age group and Indigenous status, Australia, 2003.
| |
Cases aged less than 5 years |
Cases aged 5 years or over |
Indigenous
(n=19) |
Non Indigenous
(n=93) |
Significance of difference* |
Indigenous
(n=76) |
Non-Indigenous
(n=452) |
Significance of difference* |
| Premature birth |
2 (11%) |
28 (30%) |
NS |
– |
– |
– |
| Congenital abnormality |
3 (16%) |
11 (12%) |
NS |
– |
– |
– |
| Asplenia |
0 |
1 (1.1%) |
– |
– |
7 (1.5%) |
– |
| Immuno-compromised |
2 (11%) |
8 (8.6%) |
NS |
7 (9%) |
98 (22%) |
p<0.05 |
| Chronic illness |
9 (47%) |
15 (16%) |
p<0.01 |
53 (70%) |
275(61%) |
NS |
Top of page
Pneumococcal serotypes causing disease in Australia
Pneumococcal serotypes were identified in 86 per cent (1,583/1,842) of the cases under enhanced surveillance in 2003. Of these, 71 per cent (1,129/1,583) of serotypes contained in the 7-valent conjugate pneumococcal vaccine and 91 per cent (1,444/1,583) were serotypes contained in the 23-valent polysaccharide pneumococcal vaccine (Table 11).
Table 11. Proportion of pneumococcal serotypes in cases of Invasive pneumococcal disease, covered by the 7-valent and 23-valent pneumococcal vaccines* by jurisdiction, Australia, 2003
| Vaccine |
ACT |
NSW |
NT |
Qld |
SA |
Tas |
Vic |
WA |
Total |
| 7v |
23/40
58% |
256/339
76% |
18/69
26% |
298/410
73% |
118/153
77% |
25/35
71% |
298/395
75% |
93/142
65% |
1,129/1,583
71% |
| 23v |
36/40
90% |
320/339
94% |
51/69
74% |
374/410
91% |
137/153
89% |
31/35
88% |
371/395
94% |
124/142
87% |
1,444/1,583
91% |
The frequency of vaccine serotypes in the conjugate and polysaccharide was further analysed in the target age groups for these vaccines and by Indigenous status (Table 12). The proportion of 7-valent conjugate vaccine serotypes was significantly lower in Indigenous children aged less than two years (34.6%) than in non-Indigenous children (88.3%, p<0.001). Similarly the proportion of 23-valent polysaccharide vaccine serotypes in Indigenous cases aged two years and above was significantly lower (69%) than in non-Indigenous cases (91%, p<0.001).
Table 12. The proportion of pneumococcal serotypes isolated from cases of invasive pneumococcal disease, which were serotypes in the 7-valent and 23-valent pneumococcal vaccine, by age and Indigenous status, Australia, 2003
| |
Number (%) serotypes in pneumococcal vaccines |
| |
Cases aged less than 2 years with serotypes in 7-valent conjugate vaccine |
Cases aged 2 years or more with serotypes in 23-valent vaccine |
| |
Indigenous |
Non-Indigenous |
Significance of difference* |
Indigenous |
Non-Indigenous |
Significance of difference* |
| ACT |
– |
3/7 (42%) |
– |
– |
30/33 (90%) |
– |
| NSW |
3/3 (100%) |
96/107 (89.7%) |
ns |
5/6 (83%) |
203/224 (90.6%) |
ns |
| NT |
0/11 (0%) |
4/4( 100%) |
p<0.001 |
26/38 (68%) |
14/16 (87.5%) |
ns |
| Qld |
1/7(14%) |
91/102 (89%) |
p<0.001 |
32/44 (73%) |
235/257 (91.4%) |
p<0.005 |
| SA |
– |
41/44 (93.2%) |
– |
0/1 (0%) |
95/108 (87.9%) |
p=0.07 |
| Tas |
– |
1/1 (100%) |
– |
– |
27/34
(79%) |
– |
| Vic |
3/3 (100%) |
63/75 (85%) |
ns |
2/4 (50%) |
294/313 (94%) |
p<0.005 |
| WA |
2/2 (100%) |
19/21 (90%) |
ns |
12/19 (63%) |
90/100 (90%) |
p<0.01 |
| Australia |
9/26 (34.6%) |
318/361 (88.3%) |
p<0.001 |
77/112 (69%) |
988/1,085
(91.3%) |
p<0.001 |
Trends in the numbers of 7-valent vaccine and non-7-valent vaccine serotypes in Indigenous and non-Indigenous cases aged less than two years between 2001 and 2003 are shown in Figure 4.
Top of page
Figure 4. Number of 7-valent vaccine and non-7-valent vaccine serotypes causing cases of invasive pneumococcal disease in A. Indigenous and B. non-Indigenous children aged less than 2 years, Australia 2001 to 2003
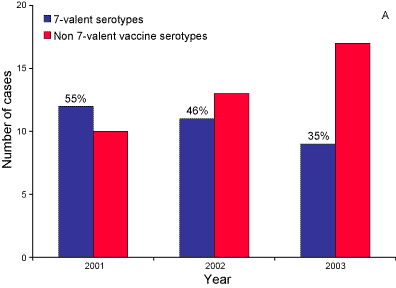
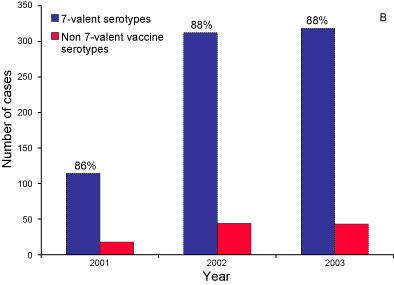
The decline in IPD due to 7-valent serotypes in Indigenous children under two years was largely seen in the Northern Territory, Queensland and Western Australia (Table 13), the jurisdictions with the largest proportion of Indigenous people.
Table 13. Pneumococcal serotypes in Indigenous children aged less than two years, 2001 to 2003, Western Australia, Queensland and Northern Territory
A. 7 valent vaccine serotypes
| |
Northern Territory |
Queensland |
Western Australia |
Total |
| 2001 |
8 |
9 |
2 |
19 |
| 2002 |
4 |
4 |
1 |
9 |
| 2003 |
0 |
1 |
2 |
3 |
B. non-7 valent vaccine serotypes
| |
Northern Territory |
Queensland |
Western Australia |
Total |
| 2001 |
4 |
0 |
6 |
10 |
| 2002 |
3 |
6 |
4 |
13 |
| 2003 |
11 |
8 |
0 |
19 |
Top of page
Vaccination status of Invasive pneumococcal disease cases
Data on pneumococcal vaccination were available for 58 per cent of the cases in 2003. Of the 1,082 cases with a vaccination history, the majority (758, 70%) was reported as unvaccinated. IPD was reported in 12 cases who had received vaccination with the 7-valent conjugate vaccine and in 161 cases who had received the 23-valent polysaccharide pneumococcal vaccine (Table 14).
Table 14. Vaccination status of Invasive pneumococcal disease cases (all serotypes), by age group and jurisdiction, Australia, 2003
A. Invasive pneumococcal disease cases aged less than 2 years
| Vaccination status |
ACT |
NSW |
NT |
Qld |
SA |
Tas |
Vic |
WA |
Total |
| Fully vaccinated for age |
0 |
0 |
6 |
5 |
1 |
0 |
0 |
0 |
12 |
| Partially vaccinated for age |
0 |
0 |
5 |
3 |
50 |
0 |
0 |
1 |
59 |
| Not vaccinated |
0 |
131 |
5 |
14 |
0 |
0 |
79 |
21 |
250 |
| Unknown |
7 |
4 |
0 |
101 |
0 |
1 |
10 |
4 |
127 |
B. Invasive pneumococcal disease cases aged 2 years or more.
| Vaccination status |
ACT |
NSW |
NT |
Qld |
SA |
Tas |
Vic |
WA |
Total |
| Fully vaccinated for age |
0 |
53 |
16 |
21 |
20 |
4 |
44 |
3 |
161 |
| Partially vaccinated for age |
0 |
1 |
5 |
6 |
0 |
0 |
3 |
1 |
16 |
| Not vaccinated |
1 |
207 |
34 |
44 |
57 |
30 |
128 |
74 |
575 |
| Unknown |
36 |
32 |
1 |
270 |
46 |
7 |
197 |
46 |
635 |
Further investigations were made of the 12 cases of IPD presumptively vaccinated with 7-valent conjugate vaccine. Of the six cases in the Northern Territory, five were infected with pneumococcal serotypes not in the 7-valent vaccine and one case had no serotype information. Similarly four cases in Queensland were infected with non-vaccine serotypes and one case had no serotype information. There was no serotype data in the South Australian case. Therefore there was no evidence for any vaccine failure with the 7-valent conjugate pneumococcal vaccine in Australia in 2003 for those fully vaccinated. (Table 15).
Table 15. Details of the cases of invasive pneumococcal disease that occurred in those fully vaccinated for age with 7-valent conjugate pneumococcal vaccines, by jurisdiction, Australia, 2003
| |
NSW |
NT |
Qld |
SA |
Tas |
Vic |
WA |
Total |
| Number |
0 |
6 |
5 |
1 |
0 |
0 |
0 |
12 |
| Age range (years) |
– |
0–1y |
0–1y |
0–1y |
– |
– |
– |
0–1 |
| Indigenous |
– |
5 |
4 |
0 |
– |
– |
– |
9 (65%) |
| Risk factors present |
– |
5/6 |
1/5 |
– |
– |
– |
– |
6
(43%) |
| 7-valent vaccination confirmed |
– |
6/6 |
5/5 |
0/1 |
– |
– |
– |
11
(78%) |
| Serotypes in 7-valent vaccine/number with known serotype |
– |
0/5 |
0/4 |
0/0 |
– |
– |
– |
7/12
(58%) |
| Number of identified vaccine failures |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
The majority of the 161 cases of IPD in recipients of the 23-valent vaccine, occurred in those with predisposing risk factors for IPD. Forty-one (27%) of the serotypes causing disease in these patients were not in 23-valent vaccine. In total 109 cases were presumptive 23-valent vaccine failures (Table 16).
Top of page
Table 16. Details of the cases of invasive pneumococcal disease that occurred in those fully vaccinated for age with 23-valent pneumococcal vaccines, by jurisdiction, Australia, 2003
| |
NSW |
NT |
Qld |
SA |
Tas |
Vic |
WA |
Total |
| Number |
53 |
16 |
21 |
20 |
4 |
44 |
3 |
161 |
| Age range (years) |
51–91 |
21–57 |
2–80 |
44–90 |
21–88 |
46–90 |
12–48 |
2–91 |
| Indigenous |
1 |
16 |
15 |
1 |
0 |
0 |
2 |
35 |
| Risk factors present |
51 |
16 |
12 |
11 |
4 |
43 |
1 |
138 |
| 23-valent vaccination confirmed?* |
53 |
16 |
21 |
0 |
0 |
44 |
1 |
135 |
| Serotypes in 23-valent vaccine/ number with known serotype |
37/43 |
8/16 |
12/21 |
11/20 |
4/5 |
37/43 |
1/3 |
110/151 |
| Number of identified vaccine failures |
37 |
8 |
12 |
11 |
4 |
37 |
0 |
109 |
Antibiotic resistance in pneumococcal cases
The antibiotic susceptibilities of 1,193 isolates to penicillin were tested in six jurisdictions and 719 isolates to ceftriaxone were tested in five jurisdictions (Table 17).
Table 17. S. pneumoniae susceptibility to penicillin and ceftriaxone, New South Wales, Northern Territory, South Australia, Queensland, Tasmania and Western Australia, by jurisdiction
| Antibiotic |
Susceptibility |
NSW |
NT |
Qld |
SA |
Tas |
WA |
Total |
| Penicillin |
Resistant |
14 |
0 |
18 |
0 |
0 |
1 |
33 |
| Intermediate |
29 |
7 |
45 |
7 |
1 |
20 |
109 |
| Susceptible |
346 |
64 |
384 |
101 |
42 |
114 |
1,051 |
| Total tested |
389 |
71 |
447 |
108 |
43 |
135 |
1,193 |
| % reduced susceptibility |
11% |
9.8% |
14.1% |
6.4% |
2.3% |
15.5% |
11.9% |
| Ceftriaxone |
Resistant |
NT |
0 |
3 |
0 |
0 |
0 |
3 |
| Intermediate |
NT |
0 |
5 |
0 |
0 |
1 |
6 |
| Susceptible |
NT |
61 |
439 |
34 |
43 |
133 |
710 |
| Total tested |
NT |
61 |
447 |
34 |
43 |
134 |
719 |
| % reduced susceptibility |
– |
0% |
1.8% |
0% |
0% |
0.7% |
1.3% |
Trends in antibiotic susceptibility were examined in three years combined data from the Northern Territory, South Australia, Tasmania and Western Australia, 2001 to 2003. There were decreases in the number of non-susceptible isolates in the Northern Territory and South Australia, while the proportion of non-susceptible isolates remained almost constant in Tasmania and Western Australia.
There was a decline in penicillin non-susceptible strains in children under five years of age over the three years and a significantly lower proportion of isolates with reduced susceptibility to penicillin compared with isolates from older cases in 2003 (Table 18). In all three years, the rates of penicillin non-susceptible strains were higher in Non-Indigenous cases compared with Indigenous cases, but these differences did not reach statistical significance. There was a small decline in the proportion of penicillin resistant isolates that were in the 7-valent vaccine (92 % in 2001 to 84% in 2003) but the change in proportion was not statistically significant.
Susceptibility to ceftriaxone was less frequently measured. The overall proportion of non-susceptible strains fell from 5.1 per cent to 0.3 per cent over the three years. This decline was seen in non-Indigenous cases and in cases aged five years and older where the changes in proportion of ceftriaxone-non-susceptible strains between 2001 and 2003 were statistically significant. There was only a single isolate of ceftriaxone non-susceptible pneumococci in 2003, which was a serogroup 1 from Western Australia in a non-Indigenous case (Table 18).
Top of page
Table 18. Characteristics of patients with non-susceptible pneumococcal isolates, Northern Territory, South Australia, Tasmania and Western Australia combined, 2001 to 2003
Penicillin |
2001 |
2002 |
2003 |
| Total number of cases with reduced susceptibility |
55 |
41 |
36 |
| Total cases tested |
464 |
539 |
357 |
| per cent non-susceptible isolates |
11.8% |
7.6% |
10.1% |
| Proportion of cases under 5 years with reduced susceptibility |
15/182
8.2% |
13/191
6.8% |
4/108
3.7% |
| Proportion of cases 5 years and older with reduced susceptibility |
40/282
14.2% |
28/348
8.0% |
32/245
13.1%* |
| Proportion of Indigenous cases with reduced susceptibility |
7/67
10.4% |
6/ 82
7.3% |
6/69
8.7% |
| Proportion of non-Indigenous cases with reduced susceptibility |
48/362
13.3% |
35/457
7.6% |
30/284
10.6% |
| Proportion of cases with serotypes in 7-valent vaccine |
49/53
92% |
31/35
88% |
30/32
94% |
| Proportion of cases in 23-valent vaccine |
53/53
100% |
35/35
100% |
34/35
97% |
| Proportion of cases with reduced susceptibility vaccinated |
2/55
3.6% |
2/41
4.8% |
3/36
8% |
Ceftriaxone |
| Total number of cases with reduced susceptibility |
17 |
9 |
1 |
| Total cases tested |
332 |
372 |
272 |
| per cent non-susceptible isolates |
5.1% |
2.4% |
0.3%** |
| Proportion of cases under 5 years with reduced susceptibility |
7/140
5% |
4/191
2.1% |
0/108
0% |
| Proportion of cases 5 years and older with reduced susceptibility |
10/192
5.2% |
5/181
2.8% |
1/164†
0.6% |
| Proportion of Indigenous cases with reduced susceptibility |
3/43
7% |
2/82
2.4% |
0/69
0% |
| Proportion of non-Indigenous cases with reduced susceptibility |
14/289
4.8% |
7/290
2.4% |
1/203†
0.5% |
| Proportion of cases with serotypes in 7-valent vaccine |
17/17
100% |
8/8
100% |
0
0% |
| Proportion of cases in 23-valent vaccine |
17/17
100% |
8/8
100% |
1
100% |
| Proportion of all cases with reduced susceptibility vaccinated |
2/17? |
0/9? |
0/1? |
Top of page
Discussion
Surveillance data in 2003 suggests a moderate impact of the 7vPCV vaccine on the incidence of IPD since the introduction of the vaccine program in Indigenous children in mid-2001. Evidence for this includes a decrease in the notification rates in Indigenous children under five years, (from 92 to 67 per 100,000 in males and 72 to 60 per 100,000 in females) and a decrease in the rate of disease caused by vaccine serotypes in Indigenous children (from 55% to 34%). A more marked effect on pneumococcal disease will be seen in Australia when a government-funded universal childhood pneumococcal vaccination program commences in January 2005.9 Continued surveillance to assess whether non7-valent vaccine serotypes will increase is supported by a suggestion of increase in the Northern jurisdiction's Indigenous <2 year old populations. Additionally, Whitney et al have observed a decreasing incidence of IPD in older adults possibly via herd immunity following universal childhood 7vPCV immunisation in the USA.10 The impact of the upcoming Australian childhood 7vPCV universal program might also provide herd immunity on other age groups—and therefore enhanced surveillance of all ages is strongly supported .
In 2005, free 23vPPV vaccine will be made available to all Australians aged 65 years and over. Vaccine 'failure' in recipients of the polysaccharide vaccine was noted in this and the two previous IPD surveillance reports.7,8 There is a need for more data on apparent vaccine failure in the vaccinated elderly to inform re-vaccination schedules. The effectiveness of the polysaccharide vaccine in preventing invasive disease has been estimated at 53 per cent, implying that 20,000 vaccinations are needed to prevent one infection.11,12 A universal vaccination program for elderly Australians should provide the vaccine coverage required to reduce the incidence of invasive disease in the elderly, which has not declined in the last three years. The need to provide re-vaccination for the elderly at high risk of IPD requires continual evaluation as there is limited data on the precise timing and effectiveness of re-vaccination. The 8th edition of the Australian Immunisation Handbook recommends re-vaccination in non-Indigenous adults aged less than 65 years with risk factors at 65 years or 10 years after the first dose, whichever comes later. Indigenous adults 15 to 49 years with risk factors* should be re-vaccinated five years after the first dose and again at age 50 or 10 years after the first re-vaccination, whichever comes later. Indigenous adults without risk factors should be re-vaccinated five years after initial vaccination.9
Ascertainment of IPD cases is necessary for effective surveillance. In Victoria a capture-recapture study in 2004 found, in addition to their 465 notified cases under enhanced surveillance there were 24 non-notified cases of IPD in 2003, largely from hospitals (M. Counahan, personal communication). This failure to notify public health authorities has been observed in the past in Australia 13 and recently in the United Kingdom.14 Under reporting of cases may also result from changes in surveillance practice and should be taken into account when interpreting the data presented in this report.
Declines in the number of pneumococcal isolates with reduced susceptibility to penicillin and ceftriaxone between 2001 and 2003 in the Northern Territory and among Indigenous cases is important in view of the antibiotic resistance developing during the past 20 years. Reduction of transmission of resistant strains through immunisation of children and lower levels of disease and therefore lower antibiotic use should reduce antibiotic resistance in pneumococcal disease. Continuing high levels of resistance among non-invasive isolates, the lower vaccine efficacy against otitis media and the potential for increased non-vaccine serotype disease make the impact of vaccination on antibiotic resistance uncertain. However it is useful to note the reduction over the past three years in those five years and older with reduced susceptibility for both penicillin and ceftriaxone and the overall proportion of non-Indigenous cases with the reduced susceptibility to ceftriaxone where the majority of these groups are not receiving the 7-valent vaccine. Some of this decrease may relate to other strategies to reduce drug resistance.
As vaccination becomes more widely implemented, concern has been expressed about the incidence of non-vaccine serotype disease increasing. While a trend in this direction may be suggested by 2002–2003 data of the under two year old vaccine eligible Indigenous children it is important that the serotypes continue to be reported in order to ascertain whether this trend will continue reflects the natural fluctuations over time. Therefore, while the overall rates of IPD continue to fall this 'replacement phenomenon' may not pose any threat to disease control, but on-going surveillance and serotyping of all invasive isolates along with anti-microbial resistance patterns is essential.
Top of page
References
1. Collignon PJ, Turnbridge JD. Antibiotic resistance in Streptococcus pneumoniae. Med J Aust 2000;173:S58–S64.
2. Turnidge JD, Bell JM, Collignon PJ. Rapidly emerging antimicrobial resistances in Streptococcus pneumoniae in Australia. Med J Aust 1999;170:152–155.
3. Craig AS, Erwin PC, Schaffner W, Elliott JA, Moore WL, Ussery XT, et al. Carriage of multidrug resistant Streptococcus pneumoniae and impact of chemoprophylaxis during an outbreak of meningitis at a day care centre. Clin Infect Dis 1999;29:1257–1264.
4. Nimmo GR, Bell JM, Collignon PJ. Fifteen years of surveillance by the Australian Group for Antimicrobial Resistance (AGAR). Comm Dis Intell 2003;27 Supplement:S47–S54.
5. Douglas RM, Miles HB. Vaccination against Streptococcus pneumoniae in childhood:lack of demonstrable benefit in young Australian children. J Infect Dis 1984;149:861–869.
6. Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J 2000;19:187–195.
7. Roche P, Krause V. Invasive pneumococcal disease in Australia, 2001. Commun Dis Intell 2002;26:505–519.
8. Roche P, Krause V, Andrews R, Carter L, Coleman D, Cook H, et al. Invasive pneumococcal disease in Australia, 2002. Comm Dis Intell 2003;27:466–477.
9. National Health and Medical Research. The Australian Immunisation Handbook. 8th edition. Canberra:NH&MRC;2003.
10. Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003;348:1737–1746.
11. Dear K, Holden J, Andrews R, Tatham D. Vaccines for preventing pneumococcal infections in adults. The Cochrane Library 2004;2.
12. Kelly H, Attia J, Andrews R, Heller RF. The number needed to vaccinate (NNV) and population estimates of the NNV:comparison of influenza and pneumococcal vaccine programmes for people aged 65 years and over. Vaccine 2004;22:2192–2198.
13. Robinson P. Meningococcal disease and the law:does non-notification really happen? Commun Dis Intell 1999;23:97–101.
14. Gjini A, Stuart JM, George RC, Nichols T, Heyderman RS. Capture-recpature analysis and pneumococcal meningitis estimates in England. Emerg Infec Dis 2004;10:87–93.
Top of page
Author affiliations
1. Surveillance Section, Department of Health and Ageing, Canberra, Australian Capital Territory
2. Centre for Disease Control, Department of Health and Community Services, Darwin, Northern Territory
3. Communicable Disease Branch, Department of Health, Sydney, New South Wales
4. Communicable Disease Surveillance, Department of Health and Human Services, Hobart, Tasmania
5. Communicable Diseases Section, Department of Human Services, Melbourne, Victoria
6. Communicable Disease Unit, Queensland Health, Brisbane, Queensland
7. Communicable Disease Control Branch, Department of Human Services, Adelaide, South Australia
8. Communicable Disease Control Branch, Department of Health, Perth, Western Australia
9. Communicable Diseases Control Unit, Department of Health and Community Care, Canberra, Australian Capital Territory
10. Microbiological Diagnostic Unit, University of Melbourne, Melbourne, Victoria
11. Queensland Health Pathology & Scientific Services, Brisbane, Queensland
12. Department of Microbiology, The Children's Hospital at Westmead, Westmead, New South Wales
Corresponding author: Dr Paul Roche, Surveillance Section, Department of Health and Ageing, GPO Box 9848 (MDP6), Canberra, ACT 2601. Telephone: +61 2 6289 8152. Facsimile: +61 2 6289 7791. Email: paul.roche@health.gov.au
This article was published in Communicable Diseases Intelligence Vol 28 No 4, December 2004.
